Abstract
It is becoming clear that structural-maintenance-of-chromosomes (SMC) complexes such as condensin and cohesin are involved in three-dimensional genome organization, yet their exact roles in functional organization remain unclear. We used chromatin interaction analysis by paired-end tag sequencing (ChIA-PET) to comprehensively identify genome-wide associations mediated by condensin and cohesin in fission yeast. We found that although cohesin and condensin often bind to the same loci, they direct different association networks and generate small and larger chromatin domains, respectively. Cohesin mediates associations between loci positioned within 100 kb of each other; condensin can drive longer-range associations. Moreover, condensin, but not cohesin, connects cell cycle–regulated genes bound by mitotic transcription factors. This study describes the different functions of condensin and cohesin in genome organization and how specific transcription factors function in condensin loading, cell cycle–dependent genome organization and mitotic chromosome organization to support faithful chromosome segregation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Misteli, T. Beyond the sequence: cellular organization of genome function. Cell 128, 787–800 (2007).
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009).
Rodley, C.D., Bertels, F., Jones, B. & O'Sullivan, J.M. Global identification of yeast chromosome interactions using genome conformation capture. Fungal Genet. Biol. 46, 879–886 (2009).
Duan, Z. et al. A three-dimensional model of the yeast genome. Nature 465, 363–367 (2010).
Tanizawa, H. et al. Mapping of long-range associations throughout the fission yeast genome reveals global genome organization linked to transcriptional regulation. Nucleic Acids Res. 38, 8164–8177 (2010).
Sexton, T. et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148, 458–472 (2012).
Hou, C., Li, L., Qin, Z.S. & Corces, V.G. Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol. Cell 48, 471–484 (2012).
Dixon, J.R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012).
Le, T.B., Imakaev, M.V., Mirny, L.A. & Laub, M.T. High-resolution mapping of the spatial organization of a bacterial chromosome. Science 342, 731–734 (2013).
Mizuguchi, T. et al. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe. Nature 516, 432–435 (2014).
Grob, S., Schmid, M.W. & Grossniklaus, U. Hi-C analysis in Arabidopsis identifies the KNOT, a structure with similarities to the flamenco locus of Drosophila. Mol. Cell 55, 678–693 (2014).
Feng, S. et al. Genome-wide Hi-C analyses in wild-type and mutants reveal high-resolution chromatin interactions in Arabidopsis. Mol. Cell 55, 694–707 (2014).
Rao, S.S. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Crane, E. et al. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature 523, 240–244 (2015).
Nora, E.P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Pope, B.D. et al. Topologically associating domains are stable units of replication-timing regulation. Nature 515, 402–405 (2014).
Li, L. et al. Widespread rearrangement of 3D chromatin organization underlies polycomb-mediated stress-induced silencing. Mol. Cell 58, 216–231 (2015).
Zuin, J. et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc. Natl. Acad. Sci. USA 111, 996–1001 (2014).
Sofueva, S. et al. Cohesin-mediated interactions organize chromosomal domain architecture. EMBO J. 32, 3119–3129 (2013).
Fullwood, M.J., Han, Y., Wei, C.L., Ruan, X. & Ruan, Y. Chromatin interaction analysis using paired-end tag sequencing. Curr. Protoc. Mol. Biol. 21, 21.15.1–21.15.25 (2010).
Li, G. et al. ChIA-PET tool for comprehensive chromatin interaction analysis with paired-end tag sequencing. Genome Biol. 11, R22 (2010).
Fullwood, M.J. et al. An oestrogen-receptor-α-bound human chromatin interactome. Nature 462, 58–64 (2009).
Handoko, L. et al. CTCF-mediated functional chromatin interactome in pluripotent cells. Nat. Genet. 43, 630–638 (2011).
Chepelev, I., Wei, G., Wangsa, D., Tang, Q. & Zhao, K. Characterization of genome-wide enhancer-promoter interactions reveals co-expression of interacting genes and modes of higher order chromatin organization. Cell Res. 22, 490–503 (2012).
Li, G. et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 (2012).
Kieffer-Kwon, K.R. et al. Interactome maps of mouse gene regulatory domains reveal basic principles of transcriptional regulation. Cell 155, 1507–1520 (2013).
DeMare, L.E. et al. The genomic landscape of cohesin-associated chromatin interactions. Genome Res. 23, 1224–1234 (2013).
Dowen, J.M. et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell 159, 374–387 (2014).
Tang, Z. et al. CTCF-mediated human 3D genome architecture reveals chromatin topology for transcription. Cell 163, 1611–1627 (2015).
Ji, X. et al. 3D chromosome regulatory landscape of human pluripotent cells. Cell Stem Cell 18, 262–275 (2016).
Hadjur, S. et al. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature 460, 410–413 (2009).
Nativio, R. et al. Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus. PLoS Genet. 5, e1000739 (2009).
Hou, C., Dale, R. & Dean, A. Cell type specificity of chromatin organization mediated by CTCF and cohesin. Proc. Natl. Acad. Sci. USA 107, 3651–3656 (2010).
Xiao, T., Wallace, J. & Felsenfeld, G. Specific sites in the C terminus of CTCF interact with the SA2 subunit of the cohesin complex and are required for cohesin-dependent insulation activity. Mol. Cell. Biol. 31, 2174–2183 (2011).
Haeusler, R.A., Pratt-Hyatt, M., Good, P.D., Gipson, T.A. & Engelke, D.R. Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes Dev. 22, 2204–2214 (2008).
Iwasaki, O., Tanaka, A., Tanizawa, H., Grewal, S.I. & Noma, K. Centromeric localization of dispersed Pol III genes in fission yeast. Mol. Biol. Cell 21, 254–265 (2010).
Tanaka, A. et al. Epigenetic regulation of condensin-mediated genome organization during the cell cycle and upon DNA damage through histone H3 lysine 56 acetylation. Mol. Cell 48, 532–546 (2012).
Iwasaki, O. et al. Interaction between TBP and condensin drives the organization and faithful segregation of mitotic chromosomes. Mol. Cell 59, 755–767 (2015).
Schmidt, C.K., Brookes, N. & Uhlmann, F. Conserved features of cohesin binding along fission yeast chromosomes. Genome Biol. 10, R52 (2009).
Dowen, J.M. et al. Multiple structural maintenance of chromosome complexes at transcriptional regulatory elements. Stem Cell Rep. 1, 371–378 (2013).
Van Bortle, K. et al. Insulator function and topological domain border strength scale with architectural protein occupancy. Genome Biol. 15, R82 (2014).
Lengronne, A. et al. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 430, 573–578 (2004).
D'Ambrosio, C. et al. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 22, 2215–2227 (2008).
Gullerova, M. & Proudfoot, N.J. Cohesin complex promotes transcriptional termination between convergent genes in S. pombe. Cell 132, 983–995 (2008).
Rustici, G. et al. Periodic gene expression program of the fission yeast cell cycle. Nat. Genet. 36, 809–817 (2004).
Takayama, Y. & Takahashi, K. Differential regulation of repeated histone genes during the fission yeast cell cycle. Nucleic Acids Res. 35, 3223–3237 (2007).
Alonso-Nuñez, M.L. et al. Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 16, 2003–2017 (2005).
Petit, C.S., Mehta, S., Roberts, R.H. & Gould, K.L. Ace2p contributes to fission yeast septin ring assembly by regulating mid2+ expression. J. Cell Sci. 118, 5731–5742 (2005).
Phanstiel, D.H., Boyle, A.P., Heidari, N. & Snyder, M.P. Mango: a bias-correcting ChIA-PET analysis pipeline. Bioinformatics 31, 3092–3098 (2015).
Heidari, N. et al. Genome-wide map of regulatory interactions in the human genome. Genome Res. 24, 1905–1917 (2014).
Kim, K.D. et al. Centromeric motion facilitates the mobility of interphase genomic regions in fission yeast. J. Cell Sci. 126, 5271–5283 (2013).
Tada, K., Susumu, H., Sakuno, T. & Watanabe, Y. Condensin association with histone H2A shapes mitotic chromosomes. Nature 474, 477–483 (2011).
Sutani, T. et al. Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 13, 2271–2283 (1999).
Tomonaga, T. et al. Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev. 14, 2757–2770 (2000).
Yamazaki, H., Tarumoto, Y. & Ishikawa, F. Tel1(ATM) and Rad3(ATR) phosphorylate the telomere protein Ccq1 to recruit telomerase and elongate telomeres in fission yeast. Genes Dev. 26, 241–246 (2012).
Chen, E.S., Saitoh, S., Yanagida, M. & Takahashi, K. A cell cycle-regulated GATA factor promotes centromeric localization of CENP-A in fission yeast. Mol. Cell 11, 175–187 (2003).
Allshire, R.C., Nimmo, E.R., Ekwall, K., Javerzat, J.P. & Cranston, G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9, 218–233 (1995).
Nakazawa, N. et al. RNA pol II transcript abundance controls condensin accumulation at mitotically up-regulated and heat-shock-inducible genes in fission yeast. Genes Cells 20, 481–499 (2015).
Sutani, T. et al. Condensin targets and reduces unwound DNA structures associated with transcription in mitotic chromosome condensation. Nat. Commun. 6, 7815 (2015).
Nakazawa, N. et al. Dissection of the essential steps for condensin accumulation at kinetochores and rDNAs during fission yeast mitosis. J. Cell Biol. 180, 1115–1131 (2008).
Hirano, M., Anderson, D.E., Erickson, H.P. & Hirano, T. Bimodal activation of SMC ATPase by intra- and inter-molecular interactions. EMBO J. 20, 3238–3250 (2001).
Yoshimura, S.H. et al. Condensin architecture and interaction with DNA: regulatory non-SMC subunits bind to the head of SMC heterodimer. Curr. Biol. 12, 508–513 (2002).
Kimura, K., Rybenkov, V.V., Crisona, N.J., Hirano, T. & Cozzarelli, N.R. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell 98, 239–248 (1999).
Bähler, J. et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 (1998).
Gadaleta, M.C., Iwasaki, O., Noguchi, C., Noma, K. & Noguchi, E. New vectors for epitope tagging and gene disruption in Schizosaccharomyces pombe. Biotechniques 55, 257–263 (2013).
Acknowledgements
We would like to thank the Wistar Institute genomics and bioinformatics facilities for high-throughput sequencing and genomic data analyses; the Wistar imaging facility for microscopic analysis; and the Yeast Genetic Resource Center (YGRC) for fission yeast strains. We also thank L. Showe, P. Lieberman and R. Locke for critically reading the manuscript, and S. Shaffer for editorial assistance. This work was supported by the G. Harold and Leila Y. Mathers Charitable Foundation and the NIH Director's New Innovator Award Program of the National Institutes of Health under award number (DP2-OD004348 to K.N.). Support for shared resources used in this study was provided by Cancer Center Support Grant (CCSG) P30CA010815 to the Wistar Institute.
Author information
Authors and Affiliations
Contributions
H.T. performed the bioinformatics analyses. O.I. performed the tethering assays. K.-D.K. performed ChIA-PET and other experiments. K.N. conceived and designed the study. All authors contributed to analyzing the data and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
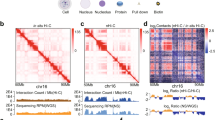
Supplementary Figure 1 Genome-wide distributions of condensin (Cut14) and cohesin (Rad21) estimated from ChIA-PET data
(a) Distribution profiles of Cut14-Pk and Rad21-Myc throughout the fission yeast genome. Positions of tRNA and 5S rRNA genes and LTRs are shown at the bottom.
(b) Venn diagram showing the overlap between Cut14 and Rad21 binding sites. Significant peaks located within same windows (100 bp) were counted as co-localization.
(c) Average binding patterns of Cut14-Pk and Rad21-Myc at tRNA and 5S rRNA genes.
(d) Average binding patterns of Rad21-Myc (top) and Cut14-Pk (bottom) at the indicated gene contexts. Convergent gene contexts were classified into two groups as depicted to the left. Arrows indicate genes. Every other combination including a divergent context was included in the ‘other’ context.
(e) ChIP results showing enrichment of Ace2-Myc at the indicated loci. The leu1 gene serves as a negative control.
(f) List of Ace2 target genes bound by condensin. Locations of Ace2 motif, CCAGCC(A/T), relative to transcriptional start sites are shown. Two potential Ace2 target genes (red) were newly identified by this study based on Ace2 binding, presence of the Ace2 motifs, and cell cycle-specific gene expression.
Supplementary Figure 2 Condensin- and cohesin-mediated chromatin domains
(a) Condensin-mediated intra-chromosomal associations between 20 kb genomic sections. Boundary indexes were calculated as described in Supplementary Methods. Boundary index, Cut14-Pk binding score, and gene annotations are shown below the association maps. Dotted lines indicate positions of predicted domain boundaries.
(b) Relation between contact probability and distances between genomic loci. Association frequencies between 100 bp genomic sections separated by same distances were used to calculate contact probabilities.
(c) Overlaps of domain boundaries estimated from Rad21 ChIA-PET and Hi-C data25.
(d) Average binding pattern of Rad21-Myc at predicted cohesin boundaries.
(e) Average boundary index score at convergent gene loci. The classes 1 and 2 and others are explained in Supplementary Fig. 1d.
(f) Relation between contact probabilities of Rad21 ChIA-PET and Hi-C data. Association frequencies between 100 bp genomic sections separated by same distances were used to calculate contact probabilities.
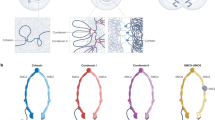
Supplementary Figure 3 Condensin- and cohesin-mediated significant gene associations.
(a,b) Classification of condensin (Cut14-Pk, a)- and cohesin (Rad21-Myc, b)-mediated associations. Numbers immediately beneath the respective genetic elements indicate numbers of condensin and cohesin association spots assigned to the indicated genetic elements. Every association was classified to the indicated combinations, and numbers in parentheses indicate observed association numbers. As a randomized simulation, every combination of association spots was categorized based on their distances, whereby each category comprises more than 400 combinations with similar distances. Every association observed in ChIA-PET data was randomly re-assigned to combinations with similar distances. This distance-conserved randomization was repeated 1000 times, and average numbers of association frequencies for respective genetic combinations were calculated. These average numbers were compared to frequencies of observed significant associations that belong to the respective genetic combinations, and their ratios are shown. There were 905 and 805 significant associations mediated by condensin and cohesin, respectively. Highly active genes are among the top 10% highest transcribed Pol II genes, and cell-cycle genes represent Ace2 and Ams2 target genes. Convergent genes indicate class 1 convergent genes.
(c) Condensin-mediated significant associations between association spots across the genome are shown at the bottom. Orange boxes represent the centromere-proximal regions (~600 kb). Heat maps of condensin-mediated associations at a 20 kb resolution and boundaries (purple lines) are shown on top.
(d) Enlarged view of condensin-mediated significant associations within the genomic region indicated by the open box in panel c.
(e) Cohesin-mediated significant associations at the indicated genomic region.
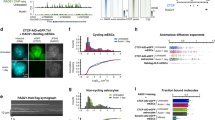
Supplementary Figure 4 Condensin-mediated associations of Ams2 target histone genes.
(a) ChIP result showing Cut14-Pk condensin enrichment at the Ams2 target genes (hta2, hht1, and hht2) during the cell cycle. The cell cycle was synchronized as described in Fig. 4a.
(b) FISH analysis co-visualizing the Ams2 target gene locus (hta1) and centromeres during the cell cycle. Representative FISH images of mitotic/M (T40) and G2 (T100) cells are shown above the graph. Nuclei were stained by DAPI (blue). The distance between two FISH foci was measured in more than 100 cells at the respective time points and binned into one of the three categories (top right).
(c) FISH analysis visualizing the indicated loci and centromeres in WT and cut14-208 condensin mutant cells during mitosis. Hydroxyurea (HU) block/release experiments were performed for the cell-cycle synchronization as diagramed at top. Exponentially growing cells were arrested in S phase by culturing in YEA medium containing 11 mM HU at 30°C for 4 hours, released by further culturing without HU for 80 minutes at the elevated temperature (36°C), because the cut14-208 mutation is temperature-sensitive6. The distance between the paired loci was summarized as described in panel b. Typical FISH images were shown below the graphs.
(d) ChIP result showing enrichment of Cut14-Pk at the Ams2 targets (hht1, hta2, and hhf1) and the leu1 gene (negative control) in WT and ams2∆ cells.
(e) FISH-IF analysis visualizing the Ace2 target (eng1) and Ams2 target genes (hht2 and hta1) with centromeres in WT and pcs1∆ mitotic cells. The c110 negative control locus is not bound by condensin. Mitotic cells were prepared by the same HU block/release procedure described in panel c. FISH analysis was accompanied by IF staining, which visualizes spindle microtubules. Mitotic cells were defined by spindle staining, and the distance between two FISH foci was measured in more than 50 mitotic cells and binned into one of the three categories.
In panels a and d, data are represented as mean ± SD.
Supplementary Figure 5 Condensin-mediated associations in mitotic cells.
(a) Summary of Cut14-Pk (condensin) binding sites in cells from asynchronous and synchronous cultures (mitotic/M and G2). Active genes are among the top 10% highest transcribed Pol II genes.
(b) Average binding patterns of Cut14-Pk at tRNA and 5S rRNA genes in the indicated cell types.
(c) Binding patterns of Cut14-Pk at the Ace2 target (mid2) and Ams2 target histone gene (hta2). Red bars indicate Ace2 and Ams2 binding motifs.
(d) IF visualization of cohesin (Rad21-Myc) and condensin (Cut14-Pk) during the cell cycle. The cell cycle was synchronized using the cdc25-22 mutation.
(e) Condensin-mediated associations between 20 kb genomic sections across the chromosome I were estimated for mitotic cells. Boundary index is shown beneath the association map. Dotted lines indicate positions of predicted domain boundaries. For comparison, the asynchronous data are also shown below.
(f) Overlaps of condensin boundaries in asynchronous and mitotic cells.
(g) Relation between contact probability and distances between genomic loci. Association frequencies between 100 bp genomic sections separated by same distances were used to calculate contact probabilities.
(h) To evaluate a similarity of the asynchronous and mitotic ChIA-PET data, the genome was divided into 20 kb bins, and association frequencies between bins were compared.
(i) Condensin-mediated significant associations between association spots across the mitotic chromosomes. Yellow indicates centromeres.
(j) Frequencies of condensin- and cohesin-mediated significant associations were plotted against distance. Associations were divided into 100 kb bins. Note that the condensin ChIA-PET data from asynchronous and mitotic cells mainly consisted of significant associations between two loci positioned more than 100 kb apart, and that the distance decays were very similar.
Supplementary Figure 6 Ace2 and Ams2 recruit condensin without involving Sep1 and Tbp1.
(a) A schematic of the tethering assay. The recruitment of Tbp1-Myc and Sep1-Myc was tested when TF (transcription factor, Ace2 or Ams2) fused to LacI-3Flag was tethered to lacO repeats at the c887 locus.
(b,c) Cells expressing Ace2 or Ams2 fused to LacI-3Flag were subjected to ChIP experiments to detect LacI-3Flag fusion proteins (blue bars), Sep1-Myc (red bars in panel b), and Tbp1-Myc (red bars in panel c) at the lacO repeats-flanking region. As a negative control (N.C.), cells carrying an empty vector were used for the same analysis. The same samples were used to detect Sep1-Myc and Tbp1-Myc at their endogenous targets, psy1 and hht2, respectively (green bars)2,3. Data are represented as mean ± SD.
Supplementary Figure 7 Stabilization of Ace2 and Ams2 proteins in the condensin mutant.
(a-c) Expression of the indicted genes in WT and cut14-208 condensin mutant cells. The WT and cut14-208 mutant were cultured at the restrictive temperature (36°C) for 2 hours. Relative expression of the Ace2 target genes (eng1 and adg2; panel a), Ams2 target genes (hht1, hhf1, hht3, hhf3, and hta2; panel b), and other cell cycle-regulated genes (ace2, ams2, slp1, and rph1; panel c) was estimated by qRT-PCR. Expression level of the leu1 gene was used as an internal control. * and ** indicate p < 0.05 and p < 0.01, respectively.
(d) WT and cut14-208 cells were cultured at 36°C for 2 hours, and cell extracts were subjected to immunoblot analysis to detect Ace2-Myc and Ams2-Myc proteins. Tubulin serves as a loading control.
(e,f) IF visualization of Ace2-Myc or Ams2-Myc proteins in WT and cut14-208 cells. Ace2-Myc and Ams2-Myc were expressed from their endogenous gene loci with their own promoters. Cells were cultured at 36°C for 2 hours and subjected to IF analysis.
(g,h) Stability of Ace2 and Ams2 proteins in WT and cut14-208 cells. Experimental procedures are depicted on top. After cycloheximide (CHX) was added to culture medium (final concentration of 100 µg/ml), Ace2-Myc (g) and Ams2-Myc (h) proteins were monitored at the indicated time points26.
(i) Schematic of the experimental procedures employed in panels j-l. The cell cycle was arrested by the HU treatment at S phase and released by further culturing without HU.
(j) Cell cycle progression was monitored in WT and cut14-208 cells by following the septation index and the percentage of dead cells caused by mitotic defects.
(k) Western blot analysis detecting Ams2-Myc proteins at the indicated time points after HU release. Equal sample loading was confirmed by Ponceau-S staining.
(l) qRT-PCR analysis estimating transcript levels of the Ams2 target genes (hht1, hhf1, and hta2) and the ams2 gene in the WT (left) and cut14-208 mutant (right).
In panels a-c and l, data are represented as mean ± SD.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Table 1 and Supplementary Note (PDF 2323 kb)
Rights and permissions
About this article
Cite this article
Kim, KD., Tanizawa, H., Iwasaki, O. et al. Transcription factors mediate condensin recruitment and global chromosomal organization in fission yeast. Nat Genet 48, 1242–1252 (2016). https://doi.org/10.1038/ng.3647
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3647
This article is cited by
-
Transcription factor Sp1 regulates mitotic chromosome assembly and segregation
Chromosoma (2022)
-
Potential roles of condensin in genome organization and beyond in fission yeast
Journal of Microbiology (2021)
-
Fission yeast condensin contributes to interphase chromatin organization and prevents transcription-coupled DNA damage
Genome Biology (2020)
-
Conserved roles of chromatin remodellers in cohesin loading onto chromatin
Current Genetics (2020)
-
Involvement of condensin in cellular senescence through gene regulation and compartmental reorganization
Nature Communications (2019)