Abstract
Genome-wide association studies have identified several loci associated with pancreatic cancer risk; however, the mechanisms by which genetic factors influence the development of sporadic pancreatic cancer remain largely unknown. Here, by using genome-wide association analysis and functional characterization, we identify a long intergenic noncoding RNA (lincRNA), LINC00673, as a potential tumor suppressor whose germline variation is associated with pancreatic cancer risk. LINC00673 is able to reinforce the interaction of PTPN11 with PRPF19, an E3 ubiquitin ligase, and promote PTPN11 degradation through ubiquitination, which causes diminished SRC–ERK oncogenic signaling and enhanced activation of the STAT1-dependent antitumor response. A G>A change at rs11655237 in exon 4 of LINC00673 creates a target site for miR-1231 binding, which diminishes the effect of LINC00673 in an allele-specific manner and thus confers susceptibility to tumorigenesis. These findings shed new light on the important role of LINC00673 in maintaining cell homeostasis and how its germline variation might confer susceptibility to pancreatic cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Pennisi, E. The Biology of Genomes. Disease risk links to gene regulation. Science 332, 1031 (2011).
Kumar, V., Wijmenga, C. & Withoff, S. From genome-wide association studies to disease mechanisms: celiac disease as a model for autoimmune diseases. Semin. Immunopathol. 34, 567–580 (2012).
Hindorff, L.A. et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl. Acad. Sci. USA 106, 9362–9367 (2009).
Cabili, M.N. et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 (2011).
Khalil, A.M. et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA 106, 11667–11672 (2009).
Rinn, J.L. et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323 (2007).
Nagano, T. et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322, 1717–1720 (2008).
Guttman, M. et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477, 295–300 (2011).
Gupta, R.A. et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076 (2010).
Li, W. et al. Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology 146, 1714–1726 (2014).
Yu, W. et al. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature 451, 202–206 (2008).
Ricaño-Ponce, I. & Wijmenga, C. Mapping of immune-mediated disease genes. Annu. Rev. Genomics Hum. Genet. 14, 325–353 (2013).
Guffanti, G. et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology 38, 3029–3038 (2013).
Jendrzejewski, J. et al. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc. Natl. Acad. Sci. USA 109, 8646–8651 (2012).
Childs, E.J. et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat. Genet. 47, 911–916 (2015).
Wu, C. et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat. Genet. 44, 62–66 (2012).
Dong, J. et al. Association analyses identify multiple new lung cancer susceptibility loci and their interactions with smoking in the Chinese population. Nat. Genet. 44, 895–899 (2012).
Panagiotou, O.A., Ioannidis, J.P. & Genome-Wide Significance Project. What should the genome-wide significance threshold be? Empirical replication of borderline genetic associations. Int. J. Epidemiol. 41, 273–286 (2012).
Wu, C. et al. Genome-wide association analyses of esophageal squamous cell carcinoma in Chinese identify multiple susceptibility loci and gene–environment interactions. Nat. Genet. 44, 1090–1097 (2012).
Badea, L., Herlea, V., Dima, S.O., Dumitrascu, T. & Popescu, I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology 55, 2016–2027 (2008).
Chen, X., Liang, H., Zhang, C.Y. & Zen, K. miRNA regulates noncoding RNA: a noncanonical function model. Trends Biochem. Sci. 37, 457–459 (2012).
Braconi, C. et al. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene 30, 4750–4756 (2011).
Paraskevopoulou, M.D. et al. DIANA-LncBase: experimentally verified and computationally predicted microRNA targets on long non-coding RNAs. Nucleic Acids Res. 41, D239–D245 (2013).
Tay, Y., Rinn, J. & Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352 (2014).
Ye, W. et al. The effect of central loops in miRNA:MRE duplexes on the efficiency of miRNA-mediated gene regulation. PLoS One 3, e1719 (2008).
Chang, T.H. et al. An enhanced computational platform for investigating the roles of regulatory RNA and for identifying functional RNA motifs. BMC Bioinformatics 14 (suppl. 2), S4 (2013).
Huang, H.Y., Chien, C.H., Jen, K.H. & Huang, H.D. RegRNA: an integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res. 34, W429–W434 (2006).
Hofacker, I.L. Vienna RNA secondary structure server. Nucleic Acids Res. 31, 3429–3431 (2003).
Kogo, R. et al. Long noncoding RNA HOTAIR regulates Polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 71, 6320–6326 (2011).
Huarte, M. et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419 (2010).
Ostman, A., Hellberg, C. & Böhmer, F.D. Protein-tyrosine phosphatases and cancer. Nat. Rev. Cancer 6, 307–320 (2006).
Maréchal, A. et al. PRP19 transforms into a sensor of RPA–ssDNA after DNA damage and drives ATR activation via a ubiquitin-mediated circuitry. Mol. Cell 53, 235–246 (2014).
Grote, M. et al. Molecular architecture of the human Prp19/CDC5L complex. Mol. Cell. Biol. 30, 2105–2119 (2010).
Vander Kooi, C.W. et al. The Prp19 U-box crystal structure suggests a common dimeric architecture for a class of oligomeric E3 ubiquitin ligases. Biochemistry 45, 121–130 (2006).
Chiarle, R., Voena, C., Ambrogio, C., Piva, R. & Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Rev. Cancer 8, 11–23 (2008).
El Touny, L.H. et al. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J. Clin. Invest. 124, 156–168 (2014).
Zhang, S.Q. et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13, 341–355 (2004).
Wu, T.R. et al. SHP-2 is a dual-specificity phosphatase involved in Stat1 dephosphorylation at both tyrosine and serine residues in nuclei. J. Biol. Chem. 277, 47572–47580 (2002).
Leibowitz, M.S. et al. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin. Cancer Res. 19, 798–808 (2013).
Iwaya, T. et al. Esophageal carcinosarcoma: a genetic analysis. Gastroenterology 113, 973–977 (1997).
Tse, G.M. et al. Clonal analysis of bilateral mammary carcinomas by clinical evaluation and partial allelotyping. Am. J. Clin. Pathol. 120, 168–174 (2003).
Tseng, R.C. et al. Genomewide loss of heterozygosity and its clinical associations in non small cell lung cancer. Int. J. Cancer 117, 241–247 (2005).
Yu, H. et al. Identification and validation of long noncoding RNA biomarkers in human non-small-cell lung carcinomas. J. Thorac. Oncol. 10, 645–654 (2015).
Gibb, E.A., Brown, C.J. & Lam, W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer 10, 38 (2011).
Tsai, M.C., Spitale, R.C. & Chang, H.Y. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 71, 3–7 (2011).
Tahira, A.C. et al. Long noncoding intronic RNAs are differentially expressed in primary and metastatic pancreatic cancer. Mol. Cancer 10, 141 (2011).
Zhou, C. et al. A miR-1231 binding site polymorphism in the 3 UTR of IFNAR1 is associated with hepatocellular carcinoma susceptibility. Gene 507, 95–98 (2012).
Kallen, A.N. et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 52, 101–112 (2013).
Grillari, J. et al. SNEV is an evolutionarily conserved splicing factor whose oligomerization is necessary for spliceosome assembly. Nucleic Acids Res. 33, 6868–6883 (2005).
Hatakeyama, S. & Nakayama, K.I. U-box proteins as a new family of ubiquitin ligases. Biochem. Biophys. Res. Commun. 302, 635–645 (2003).
Aravind, L. & Koonin, E.V. The U box is a modified RING finger—a common domain in ubiquitination. Curr. Biol. 10, R132–R134 (2000).
Yamada, T. et al. The U-box-type ubiquitin ligase PRP19β regulates astrocyte differentiation via ubiquitination of PTP1B. Brain Res. 1524, 12–25 (2013).
Zhang, J., Zhang, F. & Niu, R. Functions of Shp2 in cancer. J. Cell. Mol. Med. 19, 2075–2083 (2015).
Chan, G., Kalaitzidis, D. & Neel, B.G. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 27, 179–192 (2008).
Ren, Y. et al. Critical role of Shp2 in tumor growth involving regulation of c-Myc. Genes Cancer 1, 994–1007 (2010).
Gysin, S., Lee, S.H., Dean, N.M. & McMahon, M. Pharmacologic inhibition of RAF→MEK→ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1. Cancer Res. 65, 4870–4880 (2005).
Baron, M. & Davignon, J.L. Inhibition of IFN-γ-induced STAT1 tyrosine phosphorylation by human CMV is mediated by SHP2. J. Immunol. 181, 5530–5536 (2008).
Shuai, K. & Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3, 900–911 (2003).
Kolla, V., Lindner, D.J., Xiao, W., Borden, E.C. & Kalvakolanu, D.V. Modulation of interferon (IFN)-inducible gene expression by retinoic acid. Up-regulation of STAT1 protein in IFN-unresponsive cells. J. Biol. Chem. 271, 10508–10514 (1996).
Dimberg, A., Nilsson, K. & Oberg, F. Phosphorylation-deficient Stat1 inhibits retinoic acid–induced differentiation and cell cycle arrest in U-937 monoblasts. Blood 96, 2870–2878 (2000).
Wheeler, D.L. et al. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 31, 28–33 (2003).
Wang, L. et al. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 41, e74 (2013).
Lin, M.F., Jungreis, I. & Kellis, M. PhyloCSF: a comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics 27, i275–i282 (2011).
Schneeberger, C., Speiser, P., Kury, F. & Zeillinger, R. Quantitative detection of reverse transcriptase–PCR products by means of a novel and sensitive DNA stain. PCR Methods Appl. 4, 234–238 (1995).
Kvaratskhelia, M. & Grice, S.F. Structural analysis of protein–RNA interactions with mass spectrometry. Methods Mol. Biol. 488, 213–219 (2008).
Acknowledgements
This research was supported by the Recruitment Program of Global Youth Experts (C. Wu), grants (91229126 to D.L. and 81490753 to W.T.) from the National Natural Science Foundation of China, a grant (2014AA020609) from the National High-Tech Research and Development Program of China (C. Wu) and the Chinese Academy of Medical Sciences Intramural Funds (C. Wu, X.C. and J.Z.).
Author information
Authors and Affiliations
Contributions
D.L. conceptualized and supervised this study. C. Wu, J.Z. and X.H. contributed to the study design. C. Wu and W.T. supervised the genome-wide association analysis. J.Z. and X.H. performed and analyzed the data from most functional assays. D.Y., J.C., Z.D., L.W. and Y.H. performed sample preparation, genotyping assays and association analysis. Chengfeng Wang, X.C., Y.Z., X.M., G.J., X. Yu, X. Yang, G.C., C.Z., Z.L., Chunyou Wang, S.T.C., Y.J., X.Z. and H.S. contributed human data and samples. D.L., J.Z. and C. Wu were involved in manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
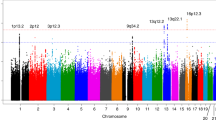
Supplementary Figure 1 Regional plots of association results and recombination rates within the significant susceptibility locus and null association between rs11655237 genotypes and survival in patients with PDAC.
(a) The negative log10-transformed P value (y axis) for each SNP is presented according to the chromosomal position of the SNP (x axis). The estimated recombination rates using 1000 Genomes Project March 2012 release samples as the reference (NCBI Build 37) are shown as light blue lines. The genomic locations of genes within the region of interest were annotated to NCBI Build 37 of the human genome assembly from the UCSC Genome Browser, and genes are shown as arrows. Both genotyped and imputed SNPs are shown, and the top SNP is labeled by rs ID. Imputation quality was estimated by masking 2% of the genotypes at random and then imputing them and comparing the imputed values with the original genotypes to estimate genotypic error rates; this yielded an error rate of 1.49% per allele. Linkage (r2) is shown for each SNP with the tagging SNP. The purple diamond represents the association of the tagging SNP identified in the GWAS stage, and the purple circle represents association of the tagging SNP in the combined genome-wide discovery and replication sample. (b) Kaplan–Meier estimates of survival time in individuals with PDAC stratified by rs11655237 (G>A) genotype. Genome-wide genotype data and survival information were available for 600 cases. After exclusion of 237 cases without disease stage information, 363 cases were available for the final analysis. The median survival time was 7.1 months. rs11655237 genotype was not significantly associated with survival. The mean survival time for individuals with the GG, GA and AA genotypes at rs11655237 were 7, 6 and 6 months, respectively, with a hazard ratio (HR) of 1.05 (95% CI = 0.89−1.25).
Supplementary Figure 2 Downregulation of LINC00673 in human cancer tissues and null association between LINC00673 expression levels and survival in patients with PDAC.
(a) LINC00673 expression is significantly lower in PDAC cells than in immortalized human pancreatic duct epithelial (HPDE6-C7) cells. RNA levels were determined by qRT–PCR; results are shown as means ± s.e.m. normalized to GAPDH. P values are from two-sided Student’s t tests. (b) LINC00673 expression, as determined by qRT–PCR, is significantly lower in PDAC tissues than in their adjacent normal tissues (n = 74). Results are shown as means ± s.e.m. normalized to GAPDH; P values are based on two-sided Student's t tests. (c) Expression levels of LINC00673 in 39 pairs of PDAC tissues and adjacent normal tissues from the GEO database (78921667). Centered values represent medians and bars represent minimum and maximum values for each group. P values are from two-sided t tests. (d,e) Downregulation of LINC00673 in human esophageal squamous cell carcinoma (ESCC) (d) and hepatocellular carcinoma (HCC) (e) in comparison with their adjacent non-tumor tissues. Results are shown as means ± s.e.m. normalized to GAPDH as determined by qRT–PCR. All P values are from two-sided t tests. (f) LINC00673 expression levels vary across different cancer types in the TCGA database. PAAD, pancreatic adenocarcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; BLCA, bladder urothelial carcinoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma. (g) Null association of LINC00673 expression, as determined by qRT–PCR, with survival in patients with PDAC. The hazard ratio (HR) was calculated using Cox regression under a log additive genetic model with age, sex and disease stage as covariates. (h) Null association of LINC00673 expression with survival in patients with PAAD in the TCGA database.
Supplementary Figure 3 Prediction of the effects of the rs11655237 G>A change on LINC00673 folding structure and interaction with miR-1231.
(a,b) Predicted folding structures and mountain plots for LINC00673 with rs11655237[G] or rs11655237[A]. Each mountain plot is an x–y graph representing the secondary structures of LINC00673, including the minimum free energy (MFE) structure, the partition function (PF) of the thermodynamic ensemble of RNA structures and the centroid structure (Centroid) in height versus position. (c) In silico prediction of interaction between miR-1231 and LINC00673 shows differences in binding within the seed region. We used three publicly available software programs, including FINDTAR3, RegRNA and RegRNA 2.0 (see URLs). (d) Relative expression levels of miR-1231 as determined by qRT–PCR in BXPC-3 and CFPAC-1 cells. Results are shown as means ± s.e.m. normalized to U6 expression. (e−g) Correlations between miR-1231 expression levels and LINC00673 expression levels as determined by qRT–PCR in normal pancreatic tissues adjacent to tumors with different rs11655237 genotypes. The r values and P values are from Pearson’s correlation analysis. (h) Copy numbers per cell of LINC00673 and miR-1231 in BXPC-3 and CFPAC-1 cells as determined by qRT–PCR. Data are shown as means ± s.e.m.
Supplementary Figure 4 Effect of LINC00673 on apoptosis, migration and invasion in pancreatic cancer cells.
(a) Relative expression levels of LINC00673 as determined by qRT–PCR in BXPC-3 and CFPAC-1 cells transfected with LINC00673[G] or LINC00673-targeting shRNAs. Results are shown as means ± s.e.m. relative to lentiviral vector control. (b) Effect of LINC00673 on apoptosis in BXPC-3 and CFPAC-1 cells as detected by flow cytometry. Results are shown as means ± s.e.m. from three experiments, each with six replications. No significant difference was found between cells transfected with LINC00673[G] and those with lentiviral vector. (c) Representative visual fields of BXPC-3 and CFPAC-1 cells treated with LINC00673[G], LINC00673[A] or shRNA to LINC00673 as compared with control cells with empty vector. Scale bars, 100 μm. (d,e) Cell counts under the microscope at 100× magnification. Data are shown as means ± s.e.m. from three random fields. The experiments were performed in triplicate. P values are from two-sided t tests.
Supplementary Figure 6 Effects of LINC00673 on the phenotypes of immortalized human pancreatic duct epithelial (HPDE6-C7) cells.
(a) Relative expression levels of LINC00673 in HPDE6-C7 cells transfected with LINC00673[A], LINC00673[G] or LINC00673-targeting shRNAs as determined by qRT–PCR. Results are shown as means ± s.e.m. relative to lentiviral vector control. (b) Overexpression of LINC00673 substantially reduced HPDE6-C7 cell proliferation. (c) Knockdown of LINC00673 significantly enhanced HPDE6-C7 cell proliferation. Cells were seeded in 96-well plates after transfection with LINC00673[A], LINC00673[G], LINC00673-targeting shRNA or control lentivirus vector, and cell number was determined every 24 h for 96 h using CCK-8 assays. Results are shown as means ± s.e.m. from three experiments, each with six replicates. *P < 0.05, compared with control on the basis of two-sided Student's t tests. (d) Effect of LINC00673 overexpression or knockdown on the cell cycle progression of HPDE6-C7 cells. Results are shown as means ± s.e.m. from three experiments. (e) Effect of LINC00673 overexpression or knockdown on colony formation of HPDE6-C7 cells (left); values are colony formation ability relative to control (set to 100%) from three experiments (right). *P < 0.05, compared with control on the basis of two-sided Student's t tests. (f) Effect of LINC00673 knockdown on colony formation of HPDE6-C7 cells in soft agar. Cells (4 × 103) were cultured in soft agar medium for 6 weeks, but no colony formation was observed. (g,h) Effect of LINC00673 overexpression or knockdown on the migration and invasion of HPDE6-C7 cells. Scale bars, 100 μm. Cell counts were determined under the microscope at 100× magnification. Data are shown as means ± s.e.m. from three random fields. The experiments were performed in triplicate. P values are from two-sided Student's t tests.
Supplementary Figure 7 Effects of altered LINC00673 levels on PTPN11 ubiquitination but not PRPF19 expression.
(a,b) BXPC-3 and CFPAC-1 cells with stable overexpression or knockdown of LINC00673[G] were treated with MG132 (5 μM) for 24 h. Cell lysates were immunoprecipitated with either control IgG or antibody to ubiquitin, followed by immunoblot analysis with antibody to PTPN11. Bottom, input of cell lysates. (c) Levels of PRPF19 mRNA as determined by qRT–PCR in BXPC-3 and CFPAC-1 cells with stable overexpression or knockdown of LINC00673[G]. Results are shown means ± s.e.m. relative to GAPDH; P values were obtained from two-sided t tests. (d) Immunoblot analysis of PRPF19 protein in control BXPC-3 and CFPAC-1 cells and in cells stably overexpressing LINC00673[G].
Supplementary Figure 8 Effects of overexpression or knockdown of LINC00673[G] on RNA levels of some genes downstream of PTPN11 in BXPC-3 and CFPAC-1 cells.
RNA levels as determined by qRT–PCR are presented as means ± s.e.m. relative to GAPDH; P values were obtained from two-sided t tests.
Supplementary Figure 9 Effects of LINC00673 on cell proliferation and cell cycle progression via PTPN11.
(a,b) Proliferation profiles of control BXPC-3 and CFPAC-1 cells and cells overexpressing LINC00673[G] transiently transfected with or without pCDNA3.1-PTPN11. Values are means ± s.e.m. from three experiments, each with six replicates. *P < 0.05, compared with cells overexpressing both LINC00673[G] and PTPN11 on the basis of two-sided t tests. (c,d) Proliferation profiles of control BXPC-3 and CFPAC-1 cells and cells expressing LINC00673 shRNA transiently transfected with or without PTPN11 siRNA. Values are means ± s.e.m. from three experiments, each with six replicates. *P < 0.05, compared with cells expressing LINC00673 shRNA and transfected with PTPN11 siRNA on the basis of a two-sided t test. (e,f) Cell cycle profiles of control BXPC-3 and CFPAC-1 cells and cells overexpressing LINC00673[G] transiently transfected with or without pCDNA3.1-PTPN11 or of control cells and cells expressing LINC00673 shRNA transiently transfected with or without PTPN11.
Supplementary Figure 10 Correlation between LINC00673 levels and the levels of some genes downstream of PTPN11 in clinical pancreatic tissues.
mRNA levels were measured by qRT–PCR in PDAC tissues (n = 74) and normalized to GAPDH. The r and P values were obtained from Pearson’s correlation analysis.
Supplementary Figure 11 Characterization of full-length human LINC00673 and cellular localization of LINC00673 in PDAC cell lines.
(a,b) Representative images of the PCR products from 5′-RACE (a) and 3′-RACE (b) (left). Sequencing of PCR products identified the boundary between the universal anchor primer and LINC00673 sequences (right). (c) The nucleotide sequence of full-length human LINC00673. (d) RNA blotting of LINC00673 extracted from BXPC-3 and CFPAC-1 cells, showing its expected molecular size. (e) Relative nuclear and cytoplasmic levels of LINC00673 in PDAC cells as determined by qRT–PCR with U6 or GAPDH as a nuclear or cytoplasmic marker, respectively. Results are presented as the percent ± s.e.m. of the total level of each molecule.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1–9. (PDF 2070 kb)
Supplementary Data 1
Genotyping data for rs11655237 in replication III samples. (XLSX 27 kb)
Supplementary Data 2
Quantification overview (111 proteins). (ZIP 6257 kb)
Supplementary Data 3
Quantification overview (34 proteins). (ZIP 10143 kb)
Rights and permissions
About this article
Cite this article
Zheng, J., Huang, X., Tan, W. et al. Pancreatic cancer risk variant in LINC00673 creates a miR-1231 binding site and interferes with PTPN11 degradation. Nat Genet 48, 747–757 (2016). https://doi.org/10.1038/ng.3568
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3568
This article is cited by
-
LINC00240 in the 6p22.1 risk locus promotes gastric cancer progression through USP10-mediated DDX21 stabilization
Journal of Experimental & Clinical Cancer Research (2023)
-
Novel hypoxia-induced HIF1α-circTDRD3-positive feedback loop promotes the growth and metastasis of colorectal cancer
Oncogene (2023)
-
PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9
Cell Death & Disease (2023)
-
Risk SNP in a transcript of RP11-638I2.4 increases lncRNA–YY1 interaction and pancreatic cancer susceptibility
Archives of Toxicology (2023)
-
The effect of SNPs in lncRNA as ceRNA on the risk and prognosis of hepatocellular carcinoma
BMC Genomics (2022)