Abstract
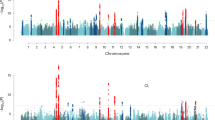
The ages of puberty, first sexual intercourse and first birth signify the onset of reproductive ability, behavior and success, respectively. In a genome-wide association study of 125,667 UK Biobank participants, we identify 38 loci associated (P < 5 × 10−8) with age at first sexual intercourse. These findings were taken forward in 241,910 men and women from Iceland and 20,187 women from the Women's Genome Health Study. Several of the identified loci also exhibit associations (P < 5 × 10−8) with other reproductive and behavioral traits, including age at first birth (variants in or near ESR1 and RBM6–SEMA3F), number of children (CADM2 and ESR1), irritable temperament (MSRA) and risk-taking propensity (CADM2). Mendelian randomization analyses infer causal influences of earlier puberty timing on earlier first sexual intercourse, earlier first birth and lower educational attainment. In turn, likely causal consequences of earlier first sexual intercourse include reproductive, educational, psychiatric and cardiometabolic outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Parent, A.S. et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 24, 668–693 (2003).
Lehmann, A., Scheffler, C. & Hermanussen, M. The variation in age at menarche: an indicator of historic developmental tempo. Anthropol. Anz. 68, 85–99 (2010).
Sohn, K. A world record in the improvement in biological standards of living in Korea: evidence from age at menarche. in Discussion Paper Series 1–34 (Centre for Economic History, Australian National University, 2015).
Waylen, A. & Wolke, D. Sex 'n' drugs 'n' rock 'n' roll: the meaning and social consequences of pubertal timing. Eur. J. Endocrinol. 151 (suppl. 3), U151–U159 (2004).
Gaudineau, A. et al. Factors associated with early menarche: results from the French Health Behaviour in School-aged Children (HBSC) study. BMC Public Health 10, 175 (2010).
Day, F.R., Elks, C.E., Murray, A., Ong, K.K. & Perry, J.R. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci. Rep. 5, 11208 (2015).
Charalampopoulos, D., McLoughlin, A., Elks, C.E. & Ong, K.K. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am. J. Epidemiol. 180, 29–40 (2014).
Gluckman, P.D. & Hanson, M.A. Evolution, development and timing of puberty. Trends Endocrinol. Metab. 17, 7–12 (2006).
Lam, T.H., Shi, H.J., Ho, L.M., Stewart, S.M. & Fan, S. Timing of pubertal maturation and heterosexual behavior among Hong Kong Chinese adolescents. Arch. Sex. Behav. 31, 359–366 (2002).
Baams, L., Dubas, J.S., Overbeek, G. & van Aken, M.A. Transitions in body and behavior: a meta-analytic study on the relationship between pubertal development and adolescent sexual behavior. J. Adolesc. Health 56, 586–598 (2015).
Hochberg, Z., Gawlik, A. & Walker, R.S. Evolutionary fitness as a function of pubertal age in 22 subsistence-based traditional societies. Int. J. Pediatr. Endocrinol. 2011, 2 (2011).
Hawes, Z.C., Wellings, K. & Stephenson, J. First heterosexual intercourse in the United Kingdom: a review of the literature. J. Sex Res. 47, 137–152 (2010).
Waldron, M. et al. Parental separation, parental alcoholism, and timing of first sexual intercourse. J. Adolesc. Health 56, 550–556 (2015).
Lenciauskiene, I. & Zaborskis, A. The effects of family structure, parent–child relationship and parental monitoring on early sexual behaviour among adolescents in nine European countries. Scand. J. Public Health 36, 607–618 (2008).
Ingham, R., Woodcock, A. & Stenner, K. Getting to know you... young people's knowledge of their partners at first intercourse. J. Community Appl. Soc. Psychol. 1, 117–132 (1991).
Allen, J.P., Schad, M.M., Oudekerk, B. & Chango, J. What ever happened to the “cool” kids? Long-term sequelae of early adolescent pseudomature behavior. Child Dev. 85, 1866–1880 (2014).
Martin, N.G., Eaves, L.J. & Eysenck, H.J. Genetical, environmental and personality factors influencing the age of first sexual intercourse in twins. J. Biosoc. Sci. 9, 91–97 (1977).
Harden, K.P. & Mendle, J. Why don't smart teens have sex? A behavioral genetic approach. Child Dev. 82, 1327–1344 (2011).
Perry, J.R. et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 514, 92–97 (2014).
Day, F.R. et al. Genetic determinants of puberty timing in men and women: shared genetic aetiology between sexes and with health-related outcomes. Nat. Commun. 6, 8842 (2015).
Burgess, S., Butterworth, A., Malarstig, A. & Thompson, S.G. Use of Mendelian randomisation to assess potential benefit of clinical intervention. Br. Med. J. 345, e7325 (2012).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Loh, P.R. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 47, 284–290 (2015).
Stulp, G., Barrett, L., Tropf, F.C. & Mills, M. Does natural selection favour taller stature among the tallest people on earth? Proc. Biol. Soc. 282, 20150211 (2015).
Sulem, P. et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat. Genet. 39, 1443–1452 (2007).
Eriksson, N. et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 6, e1000993 (2010).
Locke, A.E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
Ibrahim-Verbaas, C.A. et al. GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol. Psychiatry 21, 189–197 (2016).
Emiliani, F.E., Sedlak, T.W. & Sawa, A. Oxidative stress and schizophrenia: recent breakthroughs from an old story. Curr. Opin. Psychiatry 27, 185–190 (2014).
Ruan, H. et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. USA 99, 2748–2753 (2002).
Lahat, A. et al. Temperamental exuberance and executive function predict propensity for risk taking in childhood. Dev. Psychopathol. 24, 847–856 (2012).
Martin, A.K., Robinson, G., Dzafic, I., Reutens, D. & Mowry, B. Theory of mind and the social brain: implications for understanding the genetic basis of schizophrenia. Genes Brain Behav. 13, 104–117 (2014).
Dinzeo, T.J. & Docherty, N.M. Normal personality characteristics in schizophrenia: a review of the literature involving the FFM. J. Nerv. Ment. Dis. 195, 421–429 (2007).
Hoptman, M.J. Impulsivity and aggression in schizophrenia: a neural circuitry perspective with implications for treatment. CNS Spectr. 20, 280–286 (2015).
Altmäe, S. et al. Allelic estrogen receptor 1 (ESR1) gene variants predict the outcome of ovarian stimulation in in vitro fertilization. Mol. Hum. Reprod. 13, 521–526 (2007).
de Mattos, C.S. et al. ESR1 and ESR2 gene polymorphisms are associated with human reproduction outcomes in Brazilian women. J. Ovarian Res. 7, 114 (2014).
Drummond, A.E. The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 4, 16 (2006).
Zhang, S. et al. Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. 34, 939–980 (2013).
Hess, R.A. et al. A role for oestrogens in the male reproductive system. Nature 390, 509–512 (1997).
Carreau, S. & Hess, R.A. Oestrogens and spermatogenesis. Phil. Trans. R. Soc. Lond. B 365, 1517–1535 (2010).
Couse, J.F. et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science 286, 2328–2331 (1999).
Lee, H. et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632 (2014).
Allen, N.E., Sudlow, C., Peakman, T., Collins, R. & UK Biobank. UK Biobank data: come and get it. Sci. Transl. Med. 6, 224ed4 (2014).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Marchini, J., Howie, B., Myers, S., McVean, G. & Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 39, 906–913 (2007).
Loh, P.R. et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat. Genet. 47, 1385–1392 (2015).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Gudbjartsson, D.F. et al. Large-scale whole-genome sequencing of the Icelandic population. Nat. Genet. 47, 435–444 (2015).
Ridker, P.M. et al. Rationale, design, and methodology of the Women's Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin. Chem. 54, 249–255 (2008).
Wood, A.R. et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat. Genet. 46, 1173–1186 (2014).
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Ward, L.D. & Kellis, M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 40, D930–D934 (2012).
Acknowledgements
This research has been conducted using the UK Biobank Resource. This work was supported by the Medical Research Council (Unit Programme numbers MC_UU_12015/1 and MC_UU_12015/2).
Author information
Authors and Affiliations
Contributions
All authors had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis. F.R.D., P.M.R., K.S., K.K.O. and J.R.B.P. designed the studies. R.A.S., A.H., A.K., G.M., O.T.M., D.G., U.T. and J.E.B. were responsible for collection and generation of data. F.R.D., H.H., D.I.C., L.M.R., P.-R.L., P.S. and J.R.B.P. performed the statistical analysis; all authors contributed to the interpretation of the findings. F.R.D., K.K.O. and J.R.B.P. drafted the manuscript; all authors contributed to the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4. (PDF 7335 kb)
Supplementary Tables 1–15
Supplementary Tables 1–15. (XLSX 816 kb)
Rights and permissions
About this article
Cite this article
Day, F., Helgason, H., Chasman, D. et al. Physical and neurobehavioral determinants of reproductive onset and success. Nat Genet 48, 617–623 (2016). https://doi.org/10.1038/ng.3551
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3551
This article is cited by
-
SynCAMs in Normal Vertebrate Neural Development and Neuropsychiatric Disorders: from the Perspective of the OCAs
Molecular Neurobiology (2024)
-
Polygenic contributions to performance on the Balloon Analogue Risk Task
Molecular Psychiatry (2023)
-
Genome-wide analysis identifies genetic effects on reproductive success and ongoing natural selection at the FADS locus
Nature Human Behaviour (2023)
-
The relationships between women’s reproductive factors: a Mendelian randomisation analysis
BMC Medicine (2022)
-
Shared genetic basis between reproductive behaviors and anxiety-related disorders
Molecular Psychiatry (2022)