Abstract
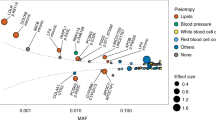
Existing knowledge of genetic variants affecting risk of coronary artery disease (CAD) is largely based on genome-wide association study (GWAS) analysis of common SNPs. Leveraging phased haplotypes from the 1000 Genomes Project, we report a GWAS meta-analysis of ∼185,000 CAD cases and controls, interrogating 6.7 million common (minor allele frequency (MAF) > 0.05) and 2.7 million low-frequency (0.005 < MAF < 0.05) variants. In addition to confirming most known CAD-associated loci, we identified ten new loci (eight additive and two recessive) that contain candidate causal genes newly implicating biological processes in vessel walls. We observed intralocus allelic heterogeneity but little evidence of low-frequency variants with larger effects and no evidence of synthetic association. Our analysis provides a comprehensive survey of the fine genetic architecture of CAD, showing that genetic susceptibility to this common disease is largely determined by common SNPs of small effect size.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
14 September 2015
In the version of this article initially published online, there was a typographical error in the third sentence of the abstract. The corrected sentence should read: "In addition to confirming most known CAD-associated loci, we identified ten new loci (eight additive and two recessive) that contain candidate causal genes newly implicating biological processes in vessel walls." The error has been corrected for the print, PDF and HTML versions of this article.
References
Kessler, T., Erdmann, J. & Schunkert, H. Genetics of coronary artery disease and myocardial infarction—2013. Curr. Cardiol. Rep. 15, 368 (2013).
O'Donnell, C.J. & Nabel, E.G. Genomics of cardiovascular disease. N. Engl. J. Med. 365, 2098–2109 (2011).
CARDIoGRAMplusC4D Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 45, 25–33 (2013).
Coronary Artery Disease Genetics (C4D) Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat. Genet. 43, 339–344 (2011).
1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Wang, F. et al. Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat. Genet. 43, 345–349 (2011).
IBC 50K CAD Consortium. Large-scale gene-centric analysis identifies novel variants for coronary artery disease. PLoS Genet. 7, e1002260 (2011).
Clarke, R. et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361, 2518–2528 (2009).
Bennet, A.M. et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. J. Am. Med. Assoc. 298, 1300–1311 (2007).
Benn, M., Nordestgaard, B.G., Grande, P., Schnohr, P. & Tybjaerg-Hansen, A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J. Am. Coll. Cardiol. 55, 2833–2842 (2010).
Cohen, J.C., Boerwinkle, E., Mosley, T.H. Jr. & Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Myocardial Infarction Genetics Consortium. A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N. Engl. J. Med. 358, 2299–2300 (2008).
Peloso, G.M. et al. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am. J. Hum. Genet. 94, 223–232 (2014).
Davies, R.W. et al. A genome-wide association study for coronary artery disease identifies a novel susceptibility locus in the major histocompatibility complex. Circ Cardiovasc Genet 5, 217–225 (2012).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Dickson, S.P., Wang, K., Krantz, I., Hakonarson, H. & Goldstein, D.B. Rare variants create synthetic genome-wide associations. PLoS Biol. 8, e1000294 (2010).
Yang, J., Lee, S.H., Goddard, M.E. & Visscher, P.M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Tang, T. et al. hNOA1 interacts with complex I and DAP3 and regulates mitochondrial respiration and apoptosis. J. Biol. Chem. 284, 5414–5424 (2009).
Chong, J.A. et al. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 80, 949–957 (1995).
Cheong, A. et al. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol. Cell 20, 45–52 (2005).
Hao, K. et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet. 8, e1003029 (2012).
Salvi, E. et al. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension 59, 248–255 (2012).
Erdmann, J. et al. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature 504, 432–436 (2013).
Casas, J.P. et al. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: a HuGE review. Am. J. Epidemiol. 164, 921–935 (2006).
Chacón-Martínez, C.A. et al. The switch-associated protein 70 (SWAP-70) bundles actin filaments and contributes to the regulation of F-actin dynamics. J. Biol. Chem. 288, 28687–28703 (2013).
Zeller, T. et al. Genetics and beyond—the transcriptome of human monocytes and disease susceptibility. PLoS ONE 5, e10693 (2010).
Fairfax, B.P. et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science 343, 1246949 (2014).
Grundberg, E. et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat. Genet. 44, 1084–1089 (2012).
Ashcroft, G.S. et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1, 260–266 (1999).
Samani, N.J. et al. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 357, 443–453 (2007).
Silvestre, J.S. et al. Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. 11, 499–506 (2005).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 (2002).
Miyata, K. et al. Elevated mature macrophage expression of human ABHD2 gene in vulnerable plaque. Biochem. Biophys. Res. Commun. 365, 207–213 (2008).
Jain, M., Bhat, G.P., Vijayraghavan, K. & Inamdar, M.S. Rudhira/BCAS3 is a cytoskeletal protein that controls Cdc42 activation and directional cell migration during angiogenesis. Exp. Cell Res. 318, 753–767 (2012).
Kim, J.Y., Ahn, H.J., Ryu, J.H., Suk, K. & Park, J.H. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1α. J. Exp. Med. 199, 113–124 (2004).
Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013).
Lango Allen, H. et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838 (2010).
Morris, A.P. et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44, 981–990 (2012).
Scott, R.A. et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat. Genet. 44, 991–1005 (2012).
Speliotes, E.K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42, 937–948 (2010).
Pearce, L.R. et al. KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell 155, 765–777 (2013).
Schork, N.J., Murray, S.S., Frazer, K.A. & Topol, E.J. Common vs. rare allele hypotheses for complex diseases. Curr. Opin. Genet. Dev. 19, 212–219 (2009).
Lettre, G., Lange, C. & Hirschhorn, J.N. Genetic model testing and statistical power in population-based association studies of quantitative traits. Genet. Epidemiol. 31, 358–362 (2007).
Do, R. et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 518, 102–106 (2015).
TG and HDL Working Group of the Exome Sequencing Project. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 371, 22–31 (2014).
Myocardial Infarction Genetics Consortium Investigators. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 371, 2072–2082 (2014).
Maurano, M.T. et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 337, 1190–1195 (2012).
Libby, P., Ridker, P.M. & Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011).
Reilly, M.P. et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet 377, 383–392 (2011).
Dichgans, M. et al. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke 45, 24–36 (2014).
Keating, B.J. et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE 3, e3583 (2008).
Voight, B.F. et al. The Metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 8, e1002793 (2012).
Schunkert, H. et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43, 333–338 (2011).
Miyata, K. et al. Increase of smooth muscle cell migration and of intimal hyperplasia in mice lacking the α/β hydrolase domain containing 2 gene. Biochem. Biophys. Res. Commun. 329, 296–304 (2005).
Bobik, A. Transforming growth factor-βs and vascular disorders. Arterioscler. Thromb. Vasc. Biol. 26, 1712–1720 (2006).
Mallat, Z. et al. Inhibition of transforming growth factor-β signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 89, 930–934 (2001).
Yang, Z. et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation 111, 2190–2197 (2005).
Aziz, M., Jacob, A., Matsuda, A. & Wang, P. Review: milk fat globule–EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis 16, 1077–1086 (2011).
Yang, J. et al. Genomic inflation factors under polygenic inheritance. Eur. J. Hum. Genet. 19, 807–812 (2011).
Howie, B., Fuchsberger, C., Stephens, M., Marchini, J. & Abecasis, G.R. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet. 44, 955–959 (2012).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
Mägi, R. & Morris, A.P. GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics 11, 288 (2010).
Cochran, W.G. The combination of estimates from different experiments. Biometrics 10, 101–129 (1954).
Higgins, J.P. & Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 (1986).
Newson, R.B. Frequentist q-values for multiple-test procedures. Stata J. 10, 568–584 (2010).
Benjamini, Y. & Yekutieli, D. The control of the false-discovery rate in multiple testing under dependency. Ann. Stat. 29, 1165–1188 (2001).
Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 44, 369–375 (2012).
So, H.C., Gui, A.H., Cherny, S.S. & Sham, P.C. Evaluating the heritability explained by known susceptibility variants: a survey of ten complex diseases. Genet. Epidemiol. 35, 310–317 (2011).
Heid, I.M. et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat. Genet. 42, 949–960 (2010).
International Consortium for Blood Pressure Genome-Wide Association Studies. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109 (2011).
Wain, L.V. et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat. Genet. 43, 1005–1011 (2011).
Welter, D. et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 42, D1001–D1006 (2014).
Fehrmann, R.S. et al. Trans-eQTLs reveal that independent genetic variants associated with a complex phenotype converge on intermediate genes, with a major role for the HLA. PLoS Genet. 7, e1002197 (2011).
Garnier, S. et al. Genome-wide haplotype analysis of cis expression quantitative trait loci in monocytes. PLoS Genet. 9, e1003240 (2013).
Gibbs, J.R. et al. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet. 6, e1000952 (2010).
Liang, L. et al. A cross-platform analysis of 14,177 expression quantitative trait loci derived from lymphoblastoid cell lines. Genome Res. 23, 716–726 (2013).
Westra, H.J. et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 45, 1238–1243 (2013).
Busch, S.J., Barnhart, R.L., Martin, G.A., Flanagan, M.A. & Jackson, R.L. Differential regulation of hepatic triglyceride lipase and 3-hydroxy-3-methylglutaryl-CoA reductase gene expression in a human hepatoma cell line, HepG2. J. Biol. Chem. 265, 22474–22479 (1990).
Park, H.J. et al. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2, 93–102 (2006).
Durrani, S., Konoplyannikov, M., Ashraf, M. & Haider, K.H. Skeletal myoblasts for cardiac repair. Regen. Med. 5, 919–932 (2010).
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Acknowledgements
We sincerely thank the participants and the medical, nursing, technical and administrative staff in each of the studies who have contributed to this project. We are grateful for support from our funders; more detailed acknowledgments are included in the Supplementary Note.
Author information
Authors and Affiliations
Consortia
Contributions
Cohort oversight: D.A., E.B., I.B.B., E.P.B., J.E.B., J.C.C., R. Collins, L.A.C., J.D., I.D., R.E., S.E.E., T.E., M.F.F., O.H.F., M.G.F., C.B.G., D. Gu, V.G., A.S.H., A. Hamsten, T.B.H., S.L.H., C.H., A. Hofman, E.I., C.I., J.W.J., P.J.K., B.-J.K., J.S.K., I.J.K., T.L., R.J.F.L., O.M., A.M., W.M., C.N.P., M.P., T.Q., D.J.R., P.M.R., S.R., R.R., V.S., D.K.S., S.M.S., U.S., A.F.S., D.J.S., J.T., P.A.Z., C.J.O'D., M.P.R., T.L.A., J.R.T., J.E., H.W., S. Kathiresan, R.M., P.D., H.S., N.J.S. and M.F. Cohort genotyping: H.-H.W., S. Kanoni, D.S., J.C.H., Jie Huang, M.E.K., Y.L., L.-P.L., A.U., S.S.A., L.B., G.D., D. Gauguier, A.H.G., M.H., B.-G.H., S.J., L. Lind, C.M.L., M.-L.L., P.K.M., A.P.M., M.S.N., N.L.P., J.S., K.E.S., S.T., L.W., I.B.B., J.C.C., R. Collins, M.F.F., A. Hofman, E.I., J.S.K., T.L., R.R., D.K.S., A.F.S., R. Clarke, P.D. and N.J.S. Cohort phenotyping: D.S., J.C.H., A.D., M.A., K.A., Y.K.K., E.M., L.M.R., S.S.A., F.B., G.D., P.F., A.H.G., O.G., Jianfeng Huang, T. Kessler, I.R.K., L. Lannfelt, W.L., L. Lind, C.M.L., P.K.M., N.H.M., N.M., T.M., F.-ur-R.M., A.P.M., N.L.P., A.P., L.S.R., A.R., M. Samuel, S.H.S., K.S.Z., D.A., J.E.B., J.C.C., R. Collins, R.E., C.B.G., V.G., A.S.H., A. Hamsten, S.L.H., E.I., J.W.J., P.J.K., J.S.K., I.J.K., O.M., A.M., M.P., R.R., D.K.S., A.F.S., D.J.S., P.A.Z., M.P.R., R. Clarke, S. Kathiresan, H.S. and N.J.S. Cohort data analyst: M.N., A.G., H.-H.W., L.M.H., C.W., S. Kanoni, D.S., T. Kyriakou, C.P.N., J.C.H., T.R.W., L.Z., A.D., M.A., S.M.A., K.A., A.B., D.I.C., S.C., I.F., N.F., C. Gieger, C. Grace, S.G., Jie Huang, S.-J.H., Y.K.K., M.E.K., K.W.L., X.L., Y.L., L.-P.L., E.M., A.C.M., N.P., L.Q., L.M.R., E.S., R.S., M. Scholz, A.V.S., E.T., A.U., X.Y., W. Zhang, W. Zhao, M.d.A., P.S.d.V., N.R.v.Z., M.F.F., J.R.T. and M.F. Meta-analysis: M.N., A.G., H.-H.W., L.M.H., C.P.N., J.R.T. and M.F. Variant annotation: M.N., A.G., H.-H.W., T. Kyriakou, J.C.H. and T.R.W. Manuscript drafting: M.N., A.G., H.-H.W., L.M.H., T. Kyriakou, J.C.H., H.W., S. Kathiresan, R.M., H.S., N.J.S. and M.F. Project steering committee: M.N., A.G., H.-H.W., L.M.H., S. Kanoni., J.C.H., D.I.C., M.E.K., N.R.v.Z., C.N.P., R.R., C.J.O'D., M.P.R., T.L.A., J.R.T., J.E., R. Clarke, H.W., S. Kathiresan, R.M., P.D., H.S., N.J.S. and M.F. (secretariat: J.C.H. and R. Clarke). CARDIoGRAMplusC4D executive committee: J.D., D. Gu, A. Hamsten, J.S.K., R.R., H.W., S. Kathiresan, P.D., H.S. and N.J.S.
Corresponding authors
Ethics declarations
Competing interests
The author declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Venn diagram showing case-control overlap between 1000G GWAS and Metabochip studies.
Venn diagram showing the number of cases (top) and controls (bottom) that overlap between the present 1000G GWAS meta-analysis study and the Metabochip study (Nat. Genet. 45, 25–33, 2013). There is a 57.5% overlap of our cases and a 40.1% overlap of our controls with the previously published study.
Supplementary Figure 2 A Manhattan plot summarizing the 1000 Genomes CAD additive association results.
The meta-analysis statistics have been adjusted for overdispersion (genomic control parameter = 1.18) and have been capped to P = 1 × 10−20. The genome-wide significance threshold is shown as a horizontal blue line at P < 5 × 10−8. Novel CAD loci are presented with red stacks and gene names (Table 1). Previously reported loci showing genome-wide significance are shown in brown, and those showing nominal significance (P < 0.05) in our meta-analysis are shown in blue (Supplementary Table 2).
Supplementary Figure 3 Comparing effect sizes for the MI subphenotype and the inclusive CAD phenotype.
Point estimates of effect sizes (odds ratios) are shown by open circles, with 95% confidence intervals represented by solid lines. The line of identity is shown as a dashed line. Loci showing marked differences in effect sizes are shown in blue.
Supplementary Figure 4 Heat map of the number of variants with power >90% to detect genome-wide significant association.
Heat map summarizing the number of variants that were calculated to be powered at ≥90% in the meta-analysis to detect a genome-wide significant association with an additive susceptibility variant with odds ratio (OR) = 1.3. Each cell is shaded from white to black to represent larger and smaller numbers of variants, respectively. The modal cell covers variants in the sector with 0.05 < MAF < 0.075 and imputation quality >0.95.
Supplementary Figure 5 Allele frequency analysis to identify strand flipping and data formatting issues.
Allele frequency analysis to identify systematic allele mismatching in individual studies due to strand flipping and other data formatting issues. (a) Proportion of variants that align with the 1000 Genomes phase 1 v3 training set minor allele after assignment to bins on the basis of MAF. The blue plot shows a typical analysis for studies with well-matched alleles, such that there is 100% concordance for lower-frequency alleles (MAF < 0.2) that declines to 50% for more frequent alleles. The red trace for study TH (Supplementary Table 1 of studies with study code) shows a marked discordance in allele frequencies that was resolved before inclusion in the meta-analysis. (b) Surface plot of 28 studies with 2 studies showing systematic strand flipping and further studies showing more subtly different allele frequency patterns. (c) Allele frequency analysis for data submitted to the meta-analysis (i.e., after any systematic mismatching issues had been resolved). Six studies of East and South Asian, Hispanic and African-American ancestries show MAF distortions that contrast with those of the remaining 42 European-ancestry studies.
Supplementary Figure 6 Quantile-quantile plots of the double–genomic controlled CAD meta-analysis results.
Shaded areas represent 95% confidence intervals. (a,b) Plots showing all additive and recessive results. (c,d) Plots showing additive and recessive results after removing variants from known loci. Supplementary Table 15 refers to the genomic control correction of each study before the final meta-analysis.
Supplementary Figure 7 Comparison of GCTA joint association analysis with standard multiple–logistic regression analysis in four studies.
We have investigated the accuracy of the GCTA joint association analysis by comparing the approximate GCTA results with a standard multiple–logistic regression analysis in 4 studies (MIGEN, PROCARDIS, OHGS and Interheart) for the 202 FDR variants. The figure shows scatterplots of the regression coefficients (left column), standard errors (center column) and –log10 (P values) (right column) for each variant; for each scatterplot, the x axis shows the standard multiple–logistic regression result, and the y axis shows the corresponding GCTA COJO result. The regression coefficients and standard errors for the majority (95%) of the variants are very accurately approximated as their results lie close to the line of identity (y = x) shown in red. The –log10 (P values) for the two analyses were positively correlated (0.86 < ρ < 0.93).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Note. (PDF 2221 kb)
Rights and permissions
About this article
Cite this article
the CARDIoGRAMplusC4D Consortium. A comprehensive 1000 Genomes–based genome-wide association meta-analysis of coronary artery disease. Nat Genet 47, 1121–1130 (2015). https://doi.org/10.1038/ng.3396
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3396
This article is cited by
-
Rare and common coding variants in lipid metabolism-related genes and their association with coronary artery disease
BMC Cardiovascular Disorders (2024)
-
PASTRY: achieving balanced power for detecting risk and protective minor alleles in meta-analysis of association studies with overlapping subjects
BMC Bioinformatics (2024)
-
The role of mitochondrial DNA copy number in cardiometabolic disease: a bidirectional two-sample mendelian randomization study
Cardiovascular Diabetology (2024)
-
The causal effect of Helicobacter pylori infection on coronary heart disease is mediated by the body mass index: a Mendelian randomization study
Scientific Reports (2024)
-
Sex-specific genetic architecture of blood pressure
Nature Medicine (2024)