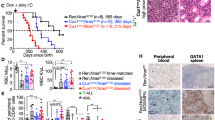
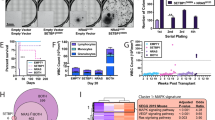
Abstract
RAS network activation is common in human cancers, and in acute myeloid leukemia (AML) this activation is achieved mainly through gain-of-function mutations in KRAS, NRAS or the receptor tyrosine kinase FLT3. We show that in mice, premalignant myeloid cells harboring a KrasG12D allele retained low levels of Ras signaling owing to negative feedback involving Spry4 that prevented transformation. In humans, SPRY4 is located on chromosome 5q, a region affected by large heterozygous deletions that are associated with aggressive disease in which gain-of-function mutations in the RAS pathway are rare. These 5q deletions often co-occur with chromosome 17 alterations involving the deletion of NF1 (another RAS negative regulator) and TP53. Accordingly, combined suppression of Spry4, Nf1 and p53 produces high levels of Ras signaling and drives AML in mice. Thus, SPRY4 is a tumor suppressor at 5q whose disruption contributes to a lethal AML subtype that appears to acquire RAS pathway activation through a loss of negative regulators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bullinger, L. et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N. Engl. J. Med. 350, 1605–1616 (2004).
Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013).
Patel, J.P. et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 366, 1079–1089 (2012).
Schubbert, S., Shannon, K. & Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 7, 295–308 (2007).
Feldser, D.M. et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 468, 572–575 (2010).
Junttila, M.R. et al. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature 468, 567–571 (2010).
Xu, J. et al. Dominant role of oncogene dosage and absence of tumor suppressor activity in Nras-driven hematopoietic transformation. Cancer Discov. 3, 993–1001 (2013).
Zhao, Z. et al. p53 loss promotes acute myeloid leukemia by enabling aberrant self-renewal. Genes Dev. 24, 1389–1402 (2010).
Braun, B.S. et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc. Natl. Acad. Sci. USA 101, 597–602 (2004).
Van Meter, M.E. et al. K-RasG12D expression induces hyperproliferation and aberrant signaling in primary hematopoietic stem/progenitor cells. Blood 109, 3945–3952 (2007).
Courtois-Cox, S. et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell 10, 459–472 (2006).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Chicas, A. et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 17, 376–387 (2010).
Kim, H.J. & Bar-Sagi, D. Modulation of signalling by Sprouty: a developing story. Nat. Rev. Mol. Cell Biol. 5, 441–450 (2004).
Chen, C. et al. MLL3 is a haploinsufficient 7q tumor suppressor in acute myeloid leukemia. Cancer Cell 25, 652–665 (2014).
Zhang, J. et al. Oncogenic Kras-induced leukemogenesis: hematopoietic stem cells as the initial target and lineage-specific progenitors as the potential targets for final leukemic transformation. Blood 113, 1304–1314 (2009).
Sabnis, A.J. et al. Oncogenic Kras initiates leukemia in hematopoietic stem cells. PLoS Biol. 7, e59 (2009).
Douet-Guilbert, N. et al. Molecular characterization of deletions of the long arm of chromosome 5 (del(5q)) in 94 MDS/AML patients. Leukemia 26, 1695–1697 (2012).
Zhao, N. et al. Molecular delineation of the smallest commonly deleted region of chromosome 5 in malignant myeloid diseases to 1–1.5 Mb and preparation of a PAC-based physical map. Proc. Natl. Acad. Sci. USA 94, 6948–6953 (1997).
Jerez, A. et al. Topography, clinical, and genomic correlates of 5q myeloid malignancies revisited. J. Clin. Oncol. 30, 1343–1349 (2012).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Bowen, D.T. et al. RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood 106, 2113–2119 (2005).
Kadia, T.M. et al. Clinical and proteomic characterization of acute myeloid leukemia with mutated RAS. Cancer 118, 5550–5559 (2012).
Scuoppo, C. et al. A tumour suppressor network relying on the polyamine-hypusine axis. Nature 487, 244–248 (2012).
Schneider, R.K. et al. Role of casein kinase 1A1 in the biology and targeted therapy of del(5q) MDS. Cancer Cell 26, 509–520 (2014).
Ebert, B.L. et al. Identification of RPS14 as a 5q− syndrome gene by RNA interference screen. Nature 451, 335–339 (2008).
Joslin, J.M. et al. Haploinsufficiency of EGR1, a candidate gene in the del(5q), leads to the development of myeloid disorders. Blood 110, 719–726 (2007).
Wang, J., Fernald, A.A., Anastasi, J., Le Beau, M.M. & Qian, Z. Haploinsufficiency of Apc leads to ineffective hematopoiesis. Blood 115, 3481–3488 (2010).
Xue, W. et al. A cluster of cooperating tumor-suppressor gene candidates in chromosomal deletions. Proc. Natl. Acad. Sci. USA 109, 8212–8217 (2012).
Davoli, T. et al. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell 155, 948–962 (2013).
Solimini, N.L. et al. Recurrent hemizygous deletions in cancers may optimize proliferative potential. Science 337, 104–109 (2012).
Cutts, B.A. et al. Nf1 deficiency cooperates with oncogenic K-RAS to induce acute myeloid leukemia in mice. Blood 114, 3629–3632 (2009).
Brennan, C.W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Dickins, R.A. et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 37, 1289–1295 (2005).
Dow, L.E. et al. A pipeline for the generation of shRNA transgenic mice. Nat. Protoc. 7, 374–393 (2012).
Fellmann, C. et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 5, 1704–1713 (2013).
Schmitt, C.A. et al. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 1, 289–298 (2002).
Hemann, M.T. et al. An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat. Genet. 33, 396–400 (2003).
Mermel, C.H. et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12, R41 (2011).
Taylor, B.S. et al. Functional copy-number alterations in cancer. PLoS One 11, e3179 (2008).
McNerney, M.E. et al. CUX1 is a haploinsufficient tumor suppressor gene on chromosome 7 frequently inactivated in acute myeloid leukemia. Blood 121, 975–983 (2013).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression levels. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
The authors gratefully acknowledge A. Chicas, O. Aksoy, C.-H. Huang, C. He and C. Chen for their expert technical assistance, advice and supportive data, as well as members of the Lowe laboratory for helpful comments. The authors also thank C. Sherr and S. Mayack for editorial comments on the manuscript. This study was supported by a Specialized Center of Research (SCOR) grant from the Leukemia & Lymphoma Society (LLS), a Starr Cancer Consortium grant, the Don Monti Memorial Research Foundation, the Geoffrey Beene Center for Cancer Research, an Award from the Ministry of Education of Taiwan to C.-C.C. and an LLS Research Fellow Award to C.D.R. (5428-15). S.W.L. is an investigator in the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Z.Z. designed, carried out and analyzed the experiments. C.-C.C. cloned miRE shRNA and carried out gene set enrichment analysis. C.-C.C. and R.S. analyzed expression patterns of TCGA data and carried out statistical analysis for all bioinformatics results. M.E.M. and M.M.L.B. provided RNA-seq, SNP array and partial karyotyping data for 35 patients from the University of Chicago. T.K. analyzed the cBioPortal database. In vivo phospho-FACS experiments were done by Z.Z. and E.D.-F. with suggestions from K.S. J.Z. cloned Spry4 shRNA–related constructs and suggested in vivo experiments. M.S.S. analyzed deletion and mutational data for SPRY4, cloned Spry4 cDNA, proposed experiments and revised the manuscript. S.C.K. characterized the phenotype and immunophenotype of models and helped to design experiments. S.W.L. designed and analyzed experiments and supervised the work. Z.Z., C.D.R. and S.W.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Lack of activated Ras signaling in non-leukemic cells harboring oncogenic Kras.
(a) Phospho-FACS analyses showing comparable levels of pErk and pS6 in bulk bone marrow (BM) population derived from recipients of LGmCreER-infected LSL-KRasG12D or control wild-type (Wt) HSPCs. (b) Quantified dot plots of a. MFI is defined as (MFI of GFP+ cells)/(MFI of GFP− cells) (n = 3–6 in each group; ns, no significant difference; two-tailed t-test). (c) Phospho-FACS analyses of BM-derived Kit+ or Mac-1+ cells showing comparable levels of pErk and pS6 from recipients of LGmCreER-infected LSL-KRasG12D, control wild-type (Wt) HSPCs. (d) Quantified dot plots of c. MFI is defined as (MFI of GFP+ cells)/(MFI of GFP− cells) (n = 3–6 in each group; ns, no significant difference; two-tailed t-test). (e) Immunoblot showing comparable level of total Kras in spleens of three independent primary recipients of KrasG12D HSPCs with Trp53 knockdown or KrasG12D HSPCs (FACS-sorted GFP+ population). (f) Flow cytometry analyses showing representative retroviral-infected HSPCs derived from Wt or LSL-KrasG12D fetal liver cells (pre-gated on GFP+ population) express myeloid marker Mac-1 but not lymphoid markers B220 or Thy1.
Supplementary Figure 2 Characterization of Spry4 shRNAs, verification of Cre-mediated recombination and status of the Trp53 locus.
(a) Western blot demonstrating knockdown of Spry4 by multiple shRNAs. Gene transduction was achieved using puromycin-selectable retroviral construct at low or high virus titer (low m.o.i. and high m.o.i., respectively) in 3T3 cells stably expressing ectopic Spry4 cDNA. (b) Kaplan-Meier curve showing overall survival of C57BL/6 mice reconstituted with KrasG12D; HSPCs with Spry4 knockdown in comparison with recipients of control HSPCs. All mice that received KrasG12D HSPCs with Spry4 knockdown developed thymic lymphoma. (c) Top, verification of Cre-mediated recombination of the LSL cassette (top band) in GFP+ cells derived from spleens of independent animals reconstituted with KrasG12D; Trp53+/– HSPCs with Ctrl knockdown (KP-C) or KrasG12D; Trp53+/– HSPCs with Spry4 knockdown (KP-S). Bottom, allele-specific PCR analysis showing persistence of the wild-type allele of Trp53 (top band) in the same samples. Control DNA (w, wild type; h, heterozygous) and no template control (ntc) also shown.
Supplementary Figure 3 Spry4 knockdown in KrasG12D; Trp53+/– HSPCs can promote histiocytic sarcoma.
(a) Immunophenotyping of two representative independent primary histiocytic sarcomas showing GFP+ spleen cells expressing Mac-1 and Gr1 but reduced B220 in comparison with control mice. (b) Kaplan-Meier curve showing survival of mice upon secondary transplantation of KrasG12D; Trp53+/– histiocytic sarcomas with Spry4 knockdown (KP-S), in comparison with recipients of KrasG12D; Trp53+/– cells with Ctrl knockdown (KP-C) (both transplanted from two independent primary histiocytic sarcomas).
Supplementary Figure 4 Analysis of TCGA data for alterations in RAS signaling–related genes in AML.
Putative copy-number alterations and mutational status of published RAS signaling–related genes, as well as RAS negative-feedback regulatory genes. Color-coding and notation were generated automatically from cBioPortal.
Supplementary Figure 5 Co-deletion of NF1 and SPRY4 in the presence of TP53 deletion and/or mutation in human AML.
(a) Co-deletion of NF1 and SPRY4 together with deletion and/or mutation of TP53 in seven AML patients from public TCGA data (number of total and overlapping samples are indicated as in b). Top, overall genomic chromosomal alterations. Amplifications in red; deletions in blue. Middle and bottom, high-resolution chromosome 5 and chromosome 17, respectively. Asterisk denotes TP53 mutation. Samples are in the same sequence in all three panels. (b) Venn diagram showing co-occurrence of TP53, SPRY4, NRAS and NF1 alterations in TCGA patients (#P = 0.1896, ***P < 0.0005, ****P < 0.0001. P values were derived from cBioPortal (see URLs).
Supplementary Figure 6 Gene expression analysis of TCGA AML data set.
(a) Venn diagram showing overlap of significantly enriched predefined pathways identified from NRAS mutant and SPRY4-deleted AML patients from TCGA dataset. Gene expression patterns in patients with mutant NRAS (n = 14) or SPRY4 deletion (n = 9; 5 of these also had NF1 deletion, but non-overlapping with RAS mutations) were compared with control AML lacking lesions involving NRAS, KRAS, FLT3 and SPRY4 (n = 80). Predefined canonical pathways (from a total of 1,320 pathways) significantly enriched (FDR < 0.05 by gene set enrichment analysis) in NRAS mutant patients were then compared with the pathways similarly derived from patients with SPRY4 deletion. There were six overlapping pathways total, five downregulated and one upregulated; P = 1.7 × 10−5. Specifically, all five significantly downregulated pathways in the NRAS mutant group overlapped with downregulated pathways in the SPRY4-deleted group (P = 1.98 × 10−6). (b) Significantly up- or downregulated genes in SPRY4-deletion patients were defined as gene set SPRY4 SIGU (570 genes) and SPRY4 SIGD (113 genes), respectively. Gene Set Enrichment Analysis (GSEA) showing positive and negative enrichment of SPRY4 SIGU and SPRY4 SIGD in patients with NRAS mutation (n = 14), respectively. (NES = 2.44, FDR q-value = 0.0 for upregulated genes, and NES = –2.46, FDR q-value = 0.0 for downregulated genes). NES, normalized enrichment score; FDR q-val, false discovery rate q-value (the probability that a gene set with a given NES represents a false-positive finding). (c) GSEA showing positive and negative enrichment of SPRY4 SIGU and SPRY4 SIGD in patients harboring mutant KRAS (n = 6), respectively. (NES = 1.76, FDR q-value = 0.0 for upregulated genes and NES = –2.13, FDR q-value = 0.0 for downregulated genes).
Supplementary Figure 7 Cooperation between suppression of Nf1 and Spry4 in Trp53−/− background.
(a) qRT-PCR analyses showing induction of Spry4 (two sets of Spry4 primers, P1 and P2) by Nf1 shRNA and induction of Nf1 by Spry4 shRNA, as well as simultaneous suppression of both genes when cells were infected with both Nf1 shRNA and Spry4 shRNA. Data were derived from two experiments using independently generated and infected HSPCs; the mean of the control (double-knockdown of Ren) was set as 1 for both Nf1 and Spry4, and the bar graph shows the mean + s.d. of the two experiments. (b) Pre-leukemic BM analyzed by phospho-FACS analysis 5 weeks post-transplantation showing enhanced pErk and pS6 in Trp53−/− cells with double knockdown of Nf1 and Spry4 when compared with singly infected Trp53−/− cells with Nf1 knockdown only from the same animal (n = 3, independent pre-leukemic mice; one-tailed t-test, with Welch's correction when applicable).
Supplementary Figure 8 Enhanced Ras signaling output by Spry4 suppression.
(a) Representative flow cytometry analysis of peripheral blood at indicated time points post-transplantation showing enrichment of cells expressing mCherry-positive (C) Nf1 shRNA and GFP-positive (G) Spry4 shRNA but no enrichment with the control shRNA combination (n = 5 for all groups). shNf1; shSpry4 mouse #3 was euthanized at week 8 because of a high AML burden. (b) Representative phospho-FACS analysis showing basal pErk and pS6 levels are increased in two independently derived primary Trp53−/− leukemias with double knockdown of Nf1 and Spry4 (pre-gated mCherry–GFP double-positive, in black line) in comparison with non-leukemic spleen (mCherry–GFP double-negative, in gray line) from the same animal. (c) qRT-PCR showing knockdown of Spry4 in FACS-sorted mCherry–GFP double-positive Trp53−/−; shNf1; shSpry4 leukemias (n = 7) compared to mCherry-positive Trp53−/− leukemias with Nf1 knockdown only (n = 2, Column Statistics; bar graph shows mean + s.e.m.).
Supplementary Figure 9 Immunophenotypic characterization of Trp53−/−; shNf1; shSpry4 leukemias.
(a) Representative immunophenotyping showing that pre-gated mCherry–GFP double-positive Trp53−/−; shNf1; shSpry4 leukemia cells (black solid line) express mature myeloid markers Gr-1 and Mac-1 and the erythrocyte marker Ter-119, but no lymphoid markers (B220 or CD3ɛ). Gray dotted line represents mCherry–GFP double-negative population from the same animal. (b) Representative immunophenotyping plots showing expansion of Lin−Kit+ myeloid progenitors in mCherry–GFP double-positive BM and spleen of Trp53−/−; shNf1; shSpry4 leukemic mice. Supplementary References 1. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013). 2. Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012). 3. Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1 and 2 (PDF 2695 kb)
Rights and permissions
About this article
Cite this article
Zhao, Z., Chen, CC., Rillahan, C. et al. Cooperative loss of RAS feedback regulation drives myeloid leukemogenesis. Nat Genet 47, 539–543 (2015). https://doi.org/10.1038/ng.3251
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3251
This article is cited by
-
Evolutionary action score identifies a subset of TP53 mutated myelodysplastic syndrome with favorable prognosis
Blood Cancer Journal (2021)
-
A model for RAS mutation patterns in cancers: finding the sweet spot
Nature Reviews Cancer (2018)
-
Therapy-related myeloid neoplasms: when genetics and environment collide
Nature Reviews Cancer (2017)
-
Loss of negative regulators amplifies RAS signaling
Nature Genetics (2015)
-
Deletion of SPRY4 is a frequent event in secondary acute myeloid leukemia
Annals of Hematology (2015)