Abstract
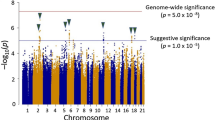
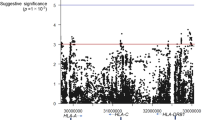
Febrile seizures represent a serious adverse event following measles, mumps and rubella (MMR) vaccination. We conducted a series of genome-wide association scans comparing children with MMR-related febrile seizures, children with febrile seizures unrelated to vaccination and controls with no history of febrile seizures. Two loci were distinctly associated with MMR-related febrile seizures, harboring the interferon-stimulated gene IFI44L (rs273259: P = 5.9 × 10−12 versus controls, P = 1.2 × 10−9 versus MMR-unrelated febrile seizures) and the measles virus receptor CD46 (rs1318653: P = 9.6 × 10−11 versus controls, P = 1.6 × 10−9 versus MMR-unrelated febrile seizures). Furthermore, four loci were associated with febrile seizures in general, implicating the sodium channel genes SCN1A (rs6432860: P = 2.2 × 10−16) and SCN2A (rs3769955: P = 3.1 × 10−10), a TMEM16 family gene (ANO3; rs114444506: P = 3.7 × 10−20) and a region associated with magnesium levels (12q21.33; rs11105468: P = 3.4 × 10−11). Finally, we show the functional relevance of ANO3 (TMEM16C) with electrophysiological experiments in wild-type and knockout rats.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barlow, W.E. et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N. Engl. J. Med. 345, 656–661 (2001).
Vestergaard, M. et al. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. J. Am. Med. Assoc. 292, 351–357 (2004).
Stafstrom, C.E. in Febrile Seizures (eds. Baram, T.Z. & Shinnar, S.) 1–25 (Academic Press, San Diego, 2002).
Millichap, J.G. & Millichap, J.J. Role of viral infections in the etiology of febrile seizures. Pediatr. Neurol. 35, 165–172 (2006).
Helbig, I., Scheffer, I.E., Mulley, J.C. & Berkovic, S.F. Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 7, 231–245 (2008).
Poduri, A. & Lowenstein, D. Epilepsy genetics—past, present, and future. Curr. Opin. Genet. Dev. 21, 325–332 (2011).
Sadleir, L.G. & Scheffer, I.E. Febrile seizures. Br. Med. J. 334, 307–311 (2007).
Hauser, W.A., Annegers, J.F., Anderson, V.E. & Kurland, L.T. The risk of seizure disorders among relatives of children with febrile convulsions. Neurology 35, 1268–1273 (1985).
Eckhaus, J. et al. Genetics of febrile seizure subtypes and syndromes: a twin study. Epilepsy Res. 105, 103–109 (2013).
Kjeldsen, M.J., Kyvik, K.O., Friis, M.L. & Christensen, K. Genetic and environmental factors in febrile seizures: a Danish population-based twin study. Epilepsy Res. 51, 167–177 (2002).
Lappalainen, T. et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501, 506–511 (2013).
Westra, H.J. et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 45, 1238–1243 (2013).
Zilliox, M.J., Parmigiani, G. & Griffin, D.E. Gene expression patterns in dendritic cells infected with measles virus compared with other pathogens. Proc. Natl. Acad. Sci. USA 103, 3363–3368 (2006).
Schoggins, J.W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Dhiman, N. et al. Variations in measles vaccine–specific humoral immunity by polymorphisms in SLAM and CD46 measles virus receptors. J. Allergy Clin. Immunol. 120, 666–672 (2007).
Kennedy, R.B. et al. Multigenic control of measles vaccine immunity mediated by polymorphisms in measles receptor, innate pathway, and cytokine genes. Vaccine 30, 2159–2167 (2012).
Clifford, H.D. et al. CD46 measles virus receptor polymorphisms influence receptor protein expression and primary measles vaccine responses in naive Australian children. Clin. Vaccine Immunol. 19, 704–710 (2012).
Ovsyannikova, I.G. et al. The association of CD46, SLAM and CD209 cellular receptor gene SNPs with variations in measles vaccine–induced immune responses: a replication study and examination of novel polymorphisms. Hum. Hered. 72, 206–223 (2011).
Kemper, C. et al. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421, 388–392 (2003).
Dörig, R.E., Marcil, A., Chopra, A. & Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75, 295–305 (1993).
Naniche, D. et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67, 6025–6032 (1993).
Ono, N. et al. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75, 4399–4401 (2001).
Thomas, E.A. et al. Heat opens axon initial segment sodium channels: a febrile seizure mechanism? Ann. Neurol. 66, 219–226 (2009).
Heron, S.E. et al. Sodium-channel defects in benign familial neonatal-infantile seizures. Lancet 360, 851–852 (2002).
Liao, Y. et al. Molecular correlates of age-dependent seizures in an inherited neonatal-infantile epilepsy. Brain 133, 1403–1414 (2010).
Schadt, E.E. et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 6, e107 (2008).
Kasperaviciute, D. et al. Epilepsy, hippocampal sclerosis and febrile seizures linked by common genetic variation around SCN1A. Brain 136, 3140–3150 (2013).
Heinzen, E.L. et al. Nova2 interacts with a cis-acting polymorphism to influence the proportions of drug-responsive splice variants of SCN1A. Am. J. Hum. Genet. 80, 876–883 (2007).
Ogiwara, I. et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 27, 5903–5914 (2007).
Rossignol, E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011, 649325 (2011).
Duran, C. & Hartzell, H.C. Physiological roles and diseases of Tmem16/Anoctamin proteins: are they all chloride channels? Acta Pharmacol. Sin. 32, 685–692 (2011).
Charlesworth, G. et al. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am. J. Hum. Genet. 91, 1041–1050 (2012).
Huang, F. et al. TMEM16C facilitates Na+-activated K+ currents in rat sensory neurons and regulates pain processing. Nat. Neurosci. 16, 1284–1290 (2013).
Meyer, T.E. et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. 6, e1001045 (2010).
Kruse, H.D., Orent, E.R. & McCollum, E.V. Studies on magnesium deficiency in animals. I. Symptomatology resulting from magnesium deprivation. J. Biol. Chem. 96, 519–539 (1932).
Hanna, S., Harrison, M., MacIntyre, I. & Fraser, R. The syndrome of magnesium deficiency in man. Lancet 2, 172–176 (1960).
Anderson, W.W., Lewis, D.V., Swartzwelder, H.S. & Wilson, W.A. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res. 398, 215–219 (1986).
Ghasemi, M. & Schachter, S.C. The NMDA receptor complex as a therapeutic target in epilepsy: a review. Epilepsy Behav. 22, 617–640 (2011).
Boulant, J.A. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 31 (suppl. 5), S157–S161 (2000).
Sugiura, Y., Ogiwara, I., Hoshi, A., Yamakawa, K. & Ugawa, Y. Different degrees of loss of function between GEFS+ and SMEI Nav1.1 missense mutants at the same residue induced by rescuable folding defects. Epilepsia 53, e111–e114 (2012).
Martin, M.S. et al. Altered function of the SCN1A voltage-gated sodium channel leads to γ-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J. Biol. Chem. 285, 9823–9834 (2010).
Turner, T.L., Cockburn, F. & Forfar, J.O. Magnesium therapy in neonatal tetany. Lancet 1, 283–284 (1977).
Euser, A.G. & Cipolla, M.J. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke 40, 1169–1175 (2009).
Yuen, A.W. & Sander, J.W. Can magnesium supplementation reduce seizures in people with epilepsy? A hypothesis. Epilepsy Res. 100, 152–156 (2012).
Abdelmalik, P.A., Politzer, N. & Carlen, P.L. Magnesium as an effective adjunct therapy for drug resistant seizures. Can. J. Neurol. Sci. 39, 323–327 (2012).
Barcia, G. et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat. Genet. 44, 1255–1259 (2012).
Heron, S.E. et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 44, 1188–1190 (2012).
Wray, N.R., Purcell, S.M. & Visscher, P.M. Synthetic associations created by rare variants do not explain most GWAS results. PLoS Biol. 9, e1000579 (2011).
Tsuboi, T. Epidemiology of febrile and afebrile convulsions in children in Japan. Neurology 34, 175–181 (1984).
Lynge, E., Sandegaard, J.L. & Rebolj, M. The Danish National Patient Register. Scand. J. Public Health 39, 30–33 (2011).
Vestergaard, M. et al. The Danish National Hospital Register is a valuable study base for epidemiologic research in febrile seizures. J. Clin. Epidemiol. 59, 61–66 (2006).
Knudsen, L.B. & Olsen, J. The Danish Medical Birth Registry. Dan. Med. Bull. 45, 320–323 (1998).
Hviid, A. Postlicensure epidemiology of childhood vaccination: the Danish experience. Expert Rev. Vaccines 5, 641–649 (2006).
Olsen, J. et al. The Danish National Birth Cohort—its background, structure and aim. Scand. J. Public Health 29, 300–307 (2001).
Pedersen, C.B., Gotzsche, H., Moller, J.O. & Mortensen, P.B. The Danish Civil Registration System. A cohort of eight million persons. Dan. Med. Bull. 53, 441–449 (2006).
Hollegaard, M.V. et al. Genome-wide scans using archived neonatal dried blood spot samples. BMC Genomics 10, 297 (2009).
1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Delaneau, O., Marchini, J. & Zagury, J.F. A linear complexity phasing method for thousands of genomes. Nat. Methods 9, 179–181 (2012).
Howie, B.N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Marchini, J. & Howie, B. Genotype imputation for genome-wide association studies. Nat. Rev. Genet. 11, 499–511 (2010).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
Higgins, J.P. & Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Willer, C.J., Li, Y. & Abecasis, G.R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Purcell, S., Cherny, S.S. & Sham, P.C. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19, 149–150 (2003).
Schwarz, J.M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575–576 (2010).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Schoggins, J.W. et al. Dengue reporter viruses reveal viral dynamics in interferon receptor–deficient mice and sensitivity to interferon effectors in vitro. Proc. Natl. Acad. Sci. USA 109, 14610–14615 (2012).
Dupuis, S. et al. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33, 388–391 (2003).
Acknowledgements
The study was partially supported by a grant from the Danish Medical Research Council (0602-01818B). Research reported in this publication was supported by US National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases grant R01AI093697 (A.H.), by NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant K01DK095031 (J.W.S.) and by NIH/National Institute of Neurological Disorders and Stroke grant R01NS069229 (L.Y.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Danish National Biobank was established with the support of major grants from the Novo Nordisk Foundation, the Danish Medical Research Council and the Lundbeck Foundation. L.Y.J. is an investigator of the Howard Hughes Medical Institute. B.F. is supported by an Oak Foundation fellowship.
Author information
Authors and Affiliations
Contributions
B.F., B.P., F.G., M.M. and A.H. designed the project and drafted the manuscript. B.P., H.S., M.V. and A.H. planned and performed register data acquisition, informatics and phenotypic characterization. B.F., F.G. and L.C. carried out the statistical genetics and bioinformatics analyses. M.V.H. and D.M.H. performed sampling, whole-genome amplification and genotyping. J.L.E. and J.W.S. designed and performed the cell-based overexpression experiments and analyzed the data. T.W., F.H. and L.Y.J. designed and performed the electrophysiology experiments and analyzed the data. All authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1, 4, 5, 7 and 8. (PDF 3303 kb)
Supplementary Tables 2, 3 and 6.
Supplementary Tables 2, 3 and 6. (XLSX 69 kb)
Rights and permissions
About this article
Cite this article
Feenstra, B., Pasternak, B., Geller, F. et al. Common variants associated with general and MMR vaccine–related febrile seizures. Nat Genet 46, 1274–1282 (2014). https://doi.org/10.1038/ng.3129
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3129
This article is cited by
-
Spatiotemporal expression patterns of genes coding for plasmalemmal chloride transporters and channels in neurological diseases
Molecular Brain (2023)
-
Augmented impulsive behavior in febrile seizure-induced mice
Toxicological Research (2023)
-
SCN1A overexpression, associated with a genomic region marked by a risk variant for a common epilepsy, raises seizure susceptibility
Acta Neuropathologica (2022)
-
Interactions of genetic variants and prenatal stress in relation to the risk for recurrent respiratory infections in children
Scientific Reports (2021)
-
Fieberkrämpfe – Diagnostik und Behandlung
Zeitschrift für Epileptologie (2021)