Abstract
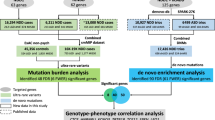
Spontaneously arising (de novo) mutations have an important role in medical genetics. For diseases with extensive locus heterogeneity, such as autism spectrum disorders (ASDs), the signal from de novo mutations is distributed across many genes, making it difficult to distinguish disease-relevant mutations from background variation. Here we provide a statistical framework for the analysis of excesses in de novo mutation per gene and gene set by calibrating a model of de novo mutation. We applied this framework to de novo mutations collected from 1,078 ASD family trios, and, whereas we affirmed a significant role for loss-of-function mutations, we found no excess of de novo loss-of-function mutations in cases with IQ above 100, suggesting that the role of de novo mutations in ASDs might reside in fundamental neurodevelopmental processes. We also used our model to identify ∼1,000 genes that are significantly lacking in functional coding variation in non-ASD samples and are enriched for de novo loss-of-function mutations identified in ASD cases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ng, S.B. et al. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat. Genet. 42, 790–793 (2010).
Iossifov, I. et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012).
Neale, B.M. et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245 (2012).
O'Roak, B.J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012).
Sanders, S.J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012).
O'Roak, B.J. et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 43, 585–589 (2011).
Antonarakis, S.E. CpG dinucleotides and human disorders. in Encyclopedia of Life Sciences (John Wiley & Sons, Chichester, UK, 2006).
Petrovski, S., Wang, Q., Heinzen, E.L., Allen, A.S. & Goldstein, D.B. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 9, e1003709 (2013).
de Ligt, J. et al. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 367, 1921–1929 (2012).
Rauch, A. et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 380, 1674–1682 (2012).
O'Roak, B.J. et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619–1622 (2012).
Betancur, C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 1380, 42–77 (2011).
Darnell, J.C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Sanders, S.J. et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70, 863–885 (2011).
Xu, B. et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat. Genet. 44, 1365–1369 (2012).
Bustamante, C.D. et al. Natural selection on protein-coding genes in the human genome. Nature 437, 1153–1157 (2005).
McDonald, J.H. & Kreitman, M. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351, 652–654 (1991).
Epi4K Consortium & Epilepsy Phenome/Genome Project. De novo mutations in epileptic encephalopathies. Nature 501, 217–221 (2013).
DePristo, M.A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Koren, A. et al. Differential relationship of DNA replication timing to different forms of human mutation and variation. Am. J. Hum. Genet. 91, 1033–1040 (2012).
Kryukov, G.V., Pennacchio, L.A. & Sunyaev, S.R. Most rare missense alleles are deleterious in humans: implications for complex disease and association studies. Am. J. Hum. Genet. 80, 727–739 (2007).
Campbell, C.D. et al. Estimating the human mutation rate using autozygosity in a founder population. Nat. Genet. 44, 1277–1281 (2012).
Conrad, D.F. et al. Variation in genome-wide mutation rates within and between human families. Nat. Genet. 43, 712–714 (2011).
Acknowledgements
All data from published studies are available in the respective publications. All newly generated data and computational tools used in this paper will be available online as downloadable material. We have also constructed a website to query genes that provides information on constraint and the de novo mutations found in the specified gene across published studies of de novo mutation. We would like to thank E. Daly and M. Chess for their contributions to data analysis and the construction of the website, respectively. We acknowledge the following resources and families who contributed to them: the National Institute of Mental Health (NIMH) repository (U24MH068457); the Autism Genetic Resource Exchange (AGRE) Consortium, a program of Autism Speaks (1U24MH081810 to C.M. Lajonchere); The Autism Simplex Collection (TASC) (grant from Autism Speaks); the Simons Foundation Autism Research Initiative (SFARI) Simplex Collection (grant from the Simons Foundation); and The Autism Consortium (grant from the Autism Consortium). This work was directly supported by US National Institutes of Health (NIH) grants R01MH089208 (M.J.D.), R01MH089025 (J.D.B.), R01MH089004 (G.D.S.), R01MH089175 (R.A.G.) and R01MH089482 (J.S.S.) and was supported in part by US NIH grants P50HD055751 (E.H.C.), R01MH057881 (B.D.) and R01MH061009 (J.S.S.). We acknowledge partial support from grants U54HG003273 (R.A.G.) and U54HG003067 (E. Lander). We thank T. Lehner (NIMH), A. Felsenfeld (National Human Genome Research Institute) and P. Bender (NIMH) for their support and contribution to the project. E.B., J.D.B., B.D., M.J.D., R.A.G., K. Roeder, A.S., G.D.S. and J.S.S. are lead investigators in the ARRA Autism Sequencing Collaboration (AASC). We would also like to thank the NHLBI GO Exome Sequencing Project (ESP) and its ongoing studies that produced and provided exome variant calls on the web: the Lung GO Sequencing Project (HL-102923), the Women's Health Initiative (WHI) Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
Author information
Authors and Affiliations
Contributions
K.E.S., B.M.N. and M.J.D. conceived and designed the mutational model and constraint methods. K.E.S. and E.B.R. executed the analyses. K.E.S., E.B.R., L.M.M., J.A.K., S.M., A.K., D.P.W., D.G.M., S.M.P., J.D.B., B.D. and K. Roeder contributed to analysis concepts and methods. K.E.S., S.J.S., C.S., A.S., K. Rehnström, S.B.G., M.D., A.P., E.B., J.D.B., E.H.C., R.A.G., G.D.S., J.S.S., B.D., K. Roeder, B.M.N. and M.J.D. contributed autism sequencing, evaluation and manuscript comments. K.E.S., E.B.R., B.M.N. and M.J.D. performed the primary writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 2 The expected and observed fraction of genes with a de novo mutation in cases and controls for four gene sets of interest.
The expected and observed overlaps between gene sets of interest and the list of genes that contain de novo mutations in unaffected individuals, autism spectrum disorder (ASD) cases and intellectual disability (ID) cases. (a) Loss-of-function mutations. (b) Missense mutations. (c) Synonymous mutations mutations. *P < 0.01; **P < 10–4.
Supplementary Figure 3 Correlation between the constraint score and RVIS.
The constraint scores (missense Z scores) determined by our method and residual variation intolerance scores from Petrovski et al.23 have a Pearson correlation of –0.35. The black line shows the linear regression between the two metrics.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 3–9 and Supplementary Note. (PDF 1521 kb)
Gene-specific probabilities of mutation.
The per-gene probabilities of mutation are listed for each gene (transcript specified) by mutation type. Probabilities of mutation are given per chromosome and have been transformed by log10. “NA” is listed when there is no probability of mutation due usually to low coverage. (XLS 3498 kb)
Top 1,003 constrained genes.
The gene-specific information listed includes transcript and identifier, chromosome, transcription start position, number of coding bases, probabilities of a synonymous and missense mutation (given per chromosome), the number of observed and expected synonymous and missense variants, the signed Z scores for the deviation for both synonymous and missense variants, and the ratio of missing missense variation (“ratio_missing”). (XLS 282 kb)
Rights and permissions
About this article
Cite this article
Samocha, K., Robinson, E., Sanders, S. et al. A framework for the interpretation of de novo mutation in human disease. Nat Genet 46, 944–950 (2014). https://doi.org/10.1038/ng.3050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3050
This article is cited by
-
Statistical methods for assessing the effects of de novo variants on birth defects
Human Genomics (2024)
-
An expanded genomic database for identifying disease-related variants
Nature (2024)
-
Genic constraint against nonsynonymous variation across the mouse genome
BMC Genomics (2023)
-
An unsupervised deep learning framework for predicting human essential genes from population and functional genomic data
BMC Bioinformatics (2023)
-
Predicting disease severity in metachromatic leukodystrophy using protein activity and a patient phenotype matrix
Genome Biology (2023)