Abstract
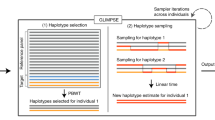
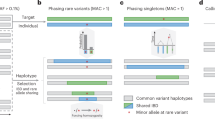
High-throughput DNA sequencing technology has transformed genetic research and is starting to make an impact on clinical practice. However, analyzing high-throughput sequencing data remains challenging, particularly in clinical settings where accuracy and turnaround times are critical. We present a new approach to this problem, implemented in a software package called Platypus. Platypus achieves high sensitivity and specificity for SNPs, indels and complex polymorphisms by using local de novo assembly to generate candidate variants, followed by local realignment and probabilistic haplotype estimation. It is an order of magnitude faster than existing tools and generates calls from raw aligned read data without preprocessing. We demonstrate the performance of Platypus in clinically relevant experimental designs by comparing with SAMtools and GATK on whole-genome and exome-capture data, by identifying de novo variation in 15 parent-offspring trios with high sensitivity and specificity, and by estimating human leukocyte antigen genotypes directly from variant calls.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
DePristo, M.A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Albers, C.A. et al. Dindel: accurate indel calls from short-read data. Genome Res. 21, 961–973 (2011).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993 (2011).
Li, R. et al. SNP detection for massively parallel whole-genome resequencing. Genome Res. 19, 1124–1132 (2009).
Iqbal, Z., Caccamo, M., Turner, I., Flicek, P. & McVean, G. De novo assembly and genotyping of variants using colored de Bruijn graphs. Nat. Genet. 44, 226–232 (2012).
Raczy, C. et al. Isaac: ultra-fast whole genome secondary analysis on Illumina sequencing platforms. Bioinformatics 29, 2041–2043 (2013).
O'Rawe, J. et al. Low concordance of multiple variant-calling pipelines: practical implications for exome and genome sequencing. Genome Med. 5, 28 (2013).
Montgomery, S.B. et al. The origin, evolution, and functional impact of short insertion-deletion variants identified in 179 human genomes. Genome Res. 23, 749–761 (2013).
Holcomb, C.L. et al. A multi-site study using high-resolution HLA genotyping by next generation sequencing. Tissue Antigens 77, 206–217 (2011).
Li, H., Ruan, J. & Durbin, R. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 18, 1851–1858 (2008).
Lunter, G. & Goodson, M. Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 21, 936–939 (2011).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, R., Li, Y., Kristiansen, K. & Wang, J. SOAP: short oligonucleotide alignment program. Bioinformatics 24, 713–714 (2008).
Garrison, A. & Marth, G. Haplotype-based variant detection from short-read sequencing, http://arxiv.org/abs/1207.3907 (2012).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Lunter, G. et al. Uncertainty in homology inferences: assessing and improving genomic sequence alignment. Genome Res. 18, 298–309 (2008).
Vinson, J.P. et al. Assembly of polymorphic genomes: algorithms and application to Ciona savignyi. Genome Res. 15, 1127–1135 (2005).
Kim, J.H., Waterman, M.S. & Li, L.M. Diploid genome reconstruction of Ciona intestinalis and comparative analysis with Ciona savignyi. Genome Res. 17, 1101–1110 (2007).
Donmez, N. & Brudno, M. in Research in Computational Molecular Biology, Lecture Notes in Computer Science Vol. 6577 (eds. Bafna, V. & Sahinalp, S.) 38–52 (Springer, Berlin, Heidelberg, 2011).
Pevzner, P.A., Tang, H. & Waterman, M.S. An Eulerian path approach to DNA fragment assembly. Proc. Natl. Acad. Sci. USA 98, 9748–9753 (2001).
Zerbino, D.R. & Birney, E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18, 821–829 (2008).
Myers, E.W. Toward simplifying and accurately formulating fragment assembly. J. Comput. Biol. 2, 275–290 (1995).
Simpson, J.T. & Durbin, R. Efficient construction of an assembly string graph using the FM-index. Bioinformatics 26, i367–i373 (2010).
Martin, H.C. et al. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum. Mol. Genet. 23, 3200–3211 (2014).
1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Kidd, J.M. et al. Characterization of missing human genome sequences and copy-number polymorphic insertions. Nat. Methods 7, 365–371 (2010).
Averof, M., Rokas, A., Wolfe, K.H. & Sharp, P.M. Evidence for a high frequency of simultaneous double-nucleotide substitutions. Science 287, 1283–1286 (2000).
McVey, M. & Lee, S.E. MMEJ repair of double-strand breaks (director's cut): deleted sequences and alternative endings. Trends Genet. 24, 529–538 (2008).
O'Roak, B.J. et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 43, 585–589 (2011).
Ku, C.S., Tan, E.K. & Cooper, D.N. From the periphery to centre stage: de novo single nucleotide variants play a key role in human genetic disease. J. Med. Genet. 50, 203–211 (2013).
Sanders, S.J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012).
Michaelson, J.J. et al. Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell 151, 1431–1442 (2012).
Veeramah, K.R. et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am. J. Hum. Genet. 90, 502–510 (2012).
Kong, A. et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature 488, 471–475 (2012).
Conrad, D.F. et al. Variation in genome-wide mutation rates within and between human families. Nat. Genet. 43, 712–714 (2011).
Chen, J.M., Ferec, C. & Cooper, D.N. Transient hypermutability, chromothripsis and replication-based mechanisms in the generation of concurrent clustered mutations. Mutat. Res. 750, 52–59 (2012).
Itoh, Y. et al. High-throughput DNA typing of HLA-A, -B, -C, and -DRB1 loci by a PCR-SSOP-Luminex method in the Japanese population. Immunogenetics 57, 717–729 (2005).
Leslie, S., Donnelly, P. & McVean, G. A statistical method for predicting classical HLA alleles from SNP data. Am. J. Hum. Genet. 82, 48–56 (2008).
de Bakker, P.I.W. et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat. Genet. 38, 1166–1172 (2006).
Ruark, E. et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature 493, 406–410 (2013).
Pagnamenta, A.T. et al. Exome sequencing can detect pathogenic mosaic mutations present at low allele frequencies. J. Hum. Genet. 57, 70–72 (2012).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115 (2012).
Koressaar, T. & Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 23, 1289–1291 (2007).
Acknowledgements
This study was funded by Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/I02593X/1 (G.L., G.M., A.R. and H.P.), by Wellcome Trust grants 102731/Z/13/Z (A.O.M.W. and S.R.F.T.), 089250/Z/09/Z (I.M.) and 090532/Z/09/Z (G.M., G.L. and A.R.), and by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme. The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), NIHR or the UK Department of Health.
Author information
Authors and Affiliations
Consortia
Contributions
A.R. developed Platypus. A.R., H.P., I.M., Z.I. and G.L. contributed code and algorithms. A.R., H.P. and G.L. analyzed data. H.P., S.R.F.T. and A.O.M.W. performed validation experiments. WGS500 contributed data. A.O.M.W., G.M. and G.L. wrote the manuscript. G.L. initiated and led the project.
Corresponding author
Ethics declarations
Competing interests
G.M. and G.L. are cofounders and shareholders of Genomics, Ltd. A.R. is currently employed by Genomics, Ltd. The other authors declare no competing financial interests.
Additional information
A list of members and affiliations appears in the Supplementary Note.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–6 and Supplementary Note. (PDF 13583 kb)
Rights and permissions
About this article
Cite this article
Rimmer, A., Phan, H., Mathieson, I. et al. Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat Genet 46, 912–918 (2014). https://doi.org/10.1038/ng.3036
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3036
This article is cited by
-
ACT-Discover: identifying karyotype heterogeneity in pancreatic cancer evolution using ctDNA
Genome Medicine (2023)
-
Shining the spotlight on the neglected: new high-quality genome assemblies as a gateway to understanding the evolution of Trypanosomatidae
BMC Genomics (2023)
-
Transformation of alignment files improves performance of variant callers for long-read RNA sequencing data
Genome Biology (2023)
-
Completing a genomic characterisation of microscopic tumour samples with copy number
BMC Bioinformatics (2023)
-
Evaluating the performance of low-frequency variant calling tools for the detection of variants from short-read deep sequencing data
Scientific Reports (2023)