Abstract
We analyzed 28 thymic epithelial tumors (TETs) using next-generation sequencing and identified a missense mutation (chromosome 7 c.74146970T>A) in GTF2I at high frequency in type A thymomas, a relatively indolent subtype. In a series of 274 TETs, we detected the GTF2I mutation in 82% of type A and 74% of type AB thymomas but rarely in the aggressive subtypes, where recurrent mutations of known cancer genes have been identified. Therefore, GTF2I mutation correlated with better survival. GTF2I β and δ isoforms were expressed in TETs, and both mutant isoforms were able to stimulate cell proliferation in vitro. Thymic carcinomas carried a higher number of mutations than thymomas (average of 43.5 and 18.4, respectively). Notably, we identified recurrent mutations of known cancer genes, including TP53, CYLD, CDKN2A, BAP1 and PBRM1, in thymic carcinomas. These findings will complement the diagnostic assessment of these tumors and also facilitate development of a molecular classification and assessment of prognosis and treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Referenced accessions
NCBI Reference Sequence
References
de Jong, W.K. et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur. J. Cancer 44, 123–130 (2008).
Travis, W.D., Brambilla, E., Muller-Hermelink, H.K. & Harris, C.C. Pathology and Genetics: Tumors of The Lung, Pleura, Thymus and Heart (IARC Press, Lyon, France, 2004).
Zucali, P.A. et al. Reproducibility of the WHO classification of thymomas: practical implications. Lung Cancer 79, 236–241 (2013).
Kelly, R.J., Petrini, I., Rajan, A., Wang, Y. & Giaccone, G. Thymic malignancies: from clinical management to targeted therapies. J. Clin. Oncol. 29, 4820–4827 (2011).
Okumura, M. et al. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen. Thorac. Cardiovasc. Surg. 56, 143–150 (2008).
Roy, A.L. Biochemistry and biology of the inducible multifunctional transcription factor TFII-I: 10 years later. Gene 492, 32–41 (2012).
King, R.W., Glotzer, M. & Kirschner, M.W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell 7, 1343–1357 (1996).
Desgranges, Z.P. et al. Inhibition of TFII-I–dependent cell cycle regulation by p53. Mol. Cell. Biol. 25, 10940–10952 (2005).
Grueneberg, D.A. et al. A multifunctional DNA-binding protein that promotes the formation of serum response factor/homeodomain complexes: identity to TFII-I. Genes Dev. 11, 2482–2493 (1997).
Ashworth, T. & Roy, A.L. Phase specific functions of the transcription factor TFII-I during cell cycle. Cell Cycle 8, 596–605 (2009).
Ge, H. et al. FusionMap: detecting fusion genes from next-generation sequencing data at base-pair resolution. Bioinformatics 27, 1922–1928 (2011).
McPherson, A. et al. deFuse: an algorithm for gene fusion discovery in tumor RNA-Seq data. PLoS Comput. Biol. 7, e1001138 (2011).
Arbajian, E. et al. A novel GTF2I/NCOA2 fusion gene emphasizes the role of NCOA2 in soft tissue angiofibroma development. Genes Chromosom. Cancer 52, 330–331 (2013).
Roy, A.L. Biochemistry and biology of the inducible multifunctional transcription factor TFII-I. Gene 274, 1–13 (2001).
Enkhmandakh, B. et al. Essential functions of the Williams-Beuren syndrome–associated TFII-I genes in embryonic development. Proc. Natl. Acad. Sci. USA 106, 181–186 (2009).
Doi-Katayama, Y. et al. Solution structure of the general transcription factor 2I domain in mouse TFII-I protein. Protein Sci. 16, 1788–1792 (2007).
Verghese, E.T. et al. Interobserver variation in the classification of thymic tumours—a multicentre study using the WHO classification system. Histopathology 53, 218–223 (2008).
Girard, N., Mornex, F., Van Houtte, P., Cordier, J.F. & van Schil, P. Thymoma: a focus on current therapeutic management. J. Thorac. Oncol. 4, 119–126 (2009).
Petrini, I. et al. Expression and mutational status of c-kit in thymic epithelial tumors. J. Thorac. Oncol. 5, 1447–1453 (2010).
Ströbel, P. et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N. Engl. J. Med. 350, 2625–2626 (2004).
Chapman, M.A. et al. Initial genome sequencing and analysis of multiple myeloma. Nature 471, 467–472 (2011).
Ley, T.J. et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456, 66–72 (2008).
Bignell, G.R. et al. Signatures of mutation and selection in the cancer genome. Nature 463, 893–898 (2010).
Petrini, I. et al. Copy number aberrations of BCL2 and CDKN2A/B identified by array-CGH in thymic epithelial tumors. Cell Death Dis. 3, e351 (2012).
Beroukhim, R. et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc. Natl. Acad. Sci. USA 104, 20007–20012 (2007).
Iafrate, A.J. et al. Detection of large-scale variation in the human genome. Nat. Genet. 36, 949–951 (2004).
Ostrovnaya, I., Nanjangud, G. & Olshen, A.B. A classification model for distinguishing copy number variants from cancer-related alterations. BMC Bioinformatics 11, 297 (2010).
Trapnell, C., Pachter, L. & Salzberg, S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Chiaromonte, F., Yap, V.B. & Miller, W. Scoring pairwise genomic sequence alignments. Pac. Symp. Biocomput. 2002, 115–126 (2002).
Bailey, J.A. et al. Recent segmental duplications in the human genome. Science 297, 1003–1007 (2002).
Jurka, J. Repbase update: a database and an electronic journal of repetitive elements. Trends Genet. 16, 418–420 (2000).
Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27, 573–580 (1999).
Koboldt, D.C. et al. VarScan: variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 25, 2283–2285 (2009).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Kumar, P., Henikoff, S. & Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Saunders, C.T. et al. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28, 1811–1817 (2012).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6, 80–92 (2012).
Case, D.A. et al. AMBER 10 User Manual (University of California, San Francisco, San Francisco, California, 2008).
Berendsen, H.J.C., Postma, J.P.M., van Gunsteren, W.F., DiNola, A. & Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684 (1984).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N-logN method for Ewald sums in large systems. J. Chem. Phys. 98, 10089 (1993).
Li, W., Zhu, T. & Guan, K.L. Transformation potential of Ras isoforms correlates with activation of phosphatidylinositol 3-kinase but not ERK. J. Biol. Chem. 279, 37398–37406 (2004).
Chen, M. et al. Enhanced growth inhibition by combined DNA methylation/HDAC inhibitors in lung tumor cells with silenced CDKN2A. Int. J. Oncol. 37, 963–971 (2010).
Petrini, I. et al. Copy number aberrations of genes regulating normal thymus development in thymic epithelial tumors. Clin. Cancer Res. 19, 1960–1971 (2013).
Zucali, P.A. et al. Insulin-like growth factor-1 receptor and phosphorylated AKT-serine 473 expression in 132 resected thymomas and thymic carcinomas. Cancer 116, 4686–4695 (2010).
Acknowledgements
This work was supported by a US National Institutes of Health National Cancer Institute intramural program and the Georgetown University Lombardi Cancer Center. We thank A. Proietti for her help in reviewing pathology slides.
Author information
Authors and Affiliations
Contributions
I.P., P.S.M., Y.W. and G.G. performed study design and writing. I.P., J.G., Y.J.Z., S.B. and S.D. performed data analysis. I.P., R.L.W., M.P., C.L. and K.J.K. performed genomic assays. I.-K.K., K.-S.P. and D.V. performed in vitro assays. M.L., G.F., P.A.Z., F.C., A.F., F.R., J.R.-C. and G.G. provided samples and collected clinical data. I.P., P.S.M., Y.W. and G.G. managed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
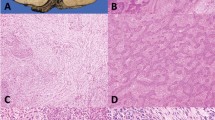
Supplementary Figure 1 Histological features of thymic epithelial tumors.
According the 2004 WHO classification a clear-cut distinction has been defined between thymomas, organotypic tumors that mimic the structure of normal thymus, and thymic carcinomas (TC), more aggressive neoplasms that do not resemble the structure of normal thymus but that of carcinomas originating in other organs. A type thymomas present bland spindle/oval epithelial tumor cells with few or no lymphocytes. Grossly, they are usually encapsulated and easily separable from the surrounding organs even in case of tumors of conspicuous dimension. Type B thymomas show epithelial cells with a predominantly round or polygonal appearance. Type B1 thymomas display tumor epithelial cells with very little atypia, scattered in a prominent population of immature non-neoplastic thymocytes that resemble the structure of normal thymus cortex. Type B2 thymomas are characterized by large polygonal epithelial tumor cells arranged in a loose network containing numerous immature T lymphocytes. B3 thymomas are composed of medium size round or polygonal epithelial tumor cells with slight atypia; these cells are mixed with a minor component of intraepithelial thymocytes. AB thymomas are composed of a lymphocyte-poor type A and a more lymphocyte-rich type B component. Thymic carcinomas (TC) are named according to their histological appearance being the squamous cell carcinoma and the undifferentiated carcinoma the most common.
Supplementary Figure 2 Arm-level copy number aberrations identify two clusters of tumors.
Using array CGH, copy number aberrations were identified. An arm level copy number aberration was defined such as one event of copy number aberration that involves more than 80% of a chromosome arm (see Online Methods for details). These aberrations defined two clusters of TETs: one with few arm level copy number aberrations and one rich in arm level copy number aberrations. These clusters trend to correlate with WHO histotypes and with the presence GTF2I mutations. There is a sub-group of samples of cluster one, to the left of the picture that presents copy number aberrations. In these samples, the copy number losses were more or equally abundant than the copy number gains, which may possibly explain the location of this subgroup in cluster 1.
Supplementary Figure 3 Frequency of copy number aberrations in thymic epithelial tumors and their WHO histotypes.
Frequency of copy number aberrations is reported for all thymic epithelial tumors in the top part of the figure; the lower part of the figure reports the data by WHO histotype. Copy number gain are in blue and copy number loss in red.
Supplementary Figure 4 Copy number aberrations at the probe-set level.
An estimation of copy number gain (blue) and loss (red) is reported for each probe in each tumor. The intensity of the colors represents the extent of copy number aberration predicted by the CGH arrays. TETs were grouped according to their histotypes.
Supplementary Figure 5 GISTIC analysis identifies significant peaks of copy number gain and loss.
GISTIC algorithm was applied to CGH data from 65 TETs in order to identify regions of copy number gain and loss that are candidate drivers of tumor growth. Significant peaks are labeled and their details are reported in the Supplementary Table 3.
Supplementary Figure 7 Representative GTF2I T>A mutation in whole exome and Sanger sequencing.
In exome sequencing (a), representative results from tumor and normal DNA of one patient, depicting the T>A mutation. The position of the mutation is shown on chromosome 7 in the top panel; the sequence of part of GTF2I exon 15 is represented in both tracks, and in the tumor track there are appreciable reads (the gray bars) carrying the mutated A. Mutated reads are not present in normal genomic material. (b) Representative GTF2I mutation in a Sanger pherogram of a type A thymoma: the forward and the reverse sequence are the top and bottom panels, respectively. The mutation T>A in forward and A/T in reverse is heterozygous and therefore identified by the presence of 2 peaks in that position.
Supplementary Figure 8 Identification of mutations in the GTF2I gene but not in the GTF2I pseudogenes.
(a) GTF2I and pseudogenes loci on chromosome 7 and their homology region. Homology regions of GTF2I and its pseudogenes are highlighted in orange. The first 12 exons of GTF2I have a unique sequence; whereas the first exons of the pseudogenes share homology sequences with GAST gene family (highlighted in light blue) that is composed of GAST, GASTL1 and GASTL2: for convenience we show only GASTL2 in the Figure. (b) GTF2I mutation (T>A) is mapped on exon 15. This region matches exon 4 of the pseudogenes, and differs by only 1 nucleotide: C in GTF2I and T in pseudogene sequences. The schema describes the allele frequencies theoretically present in a cell: one GTF2I mutated allele (1:6, ∼17%), one GTF2I WT alleles (1:6, ∼17%) and 4 pseudogenes wild type alleles (4:6, ∼67%). (c) TopoTA cloning performed in 4 tumors with GTF2I mutation. Results are reported as average of identified allele frequencies. Sequencing of cloned amplicons identified the mutation only in GTF2I but not in the pseudogenes. (d) Deep sequencing performed on 5 tumors with GTF2I T>A mutation. The mutation was found in GTF2I only and not the pseudogenes, which equals to the mutation rate of ∼17% or 1 out of 6 alleles (2 GTF2I + 4 pseudogene alleles). The mutation was not identified in the negative controls (data not shown; for details see Supplementary Table 8).
Supplementary Figure 9 GTF2I exon 15 and pseudogene exon 4 sequences, and their distribution in normal samples, tumors of GTF2I wild-type and GTF2I mutant.
(a) Sequence of GTF2I exome 15: wild type or mutant; wild type sequence of the exon 4 of the pseudogenes. The marker that distinguishes GTF2I from the pseudogenes is reported in purple (nucleotide N1). The site of mutation is reported in orange when WT and in green when mutated (nucleotide N2). (b) Distribution of GTF2I and pseudogene reads carrying the T>A mutation in normal DNA (n = 13), GTF2I WT (n = 131) and mutated tumors (n = 105) including all the samples characterized using the deep sequencing assay (n = 250). In the group of GTF2I mutated tumors, the frequency of the mutated GTF2I allele was reduced compared to the expected 17% in a population of exclusively cancer cells. This result was expected, since samples rich in non-neoplastic thymocytes were included in this group.
Supplementary Figure 10 Kaplan-Meier survival curves of patients with GTF2I-mutated (blue) and wild-type tumors (red).
(a) Thymic carcinomas (n = 33). (b) Thymomas (n = 171). The curves were compared using Log-Rank test. In thymic carcinoma the 10-year survival rate was 100% for GTF2I mutated cases (only 3 tumors) and 47% in WT tumors (n = 30). In thymomas, the 10-year survival rate was 81% and 94% for GTF2I mutated and WT tumors. (c) Estimation of the fraction of proliferating cells in thymic carcinomas, A and B3 thymomas by immunohistochemistry with an anti-Ki67 antibody performed on FFPE slides. The number of cells, positive for Ki67, was similar between WT and mutated thymomas, both in A (n = 5 and 11, respectively) and B3 histotypes (n = 17 and 8, respectively). In thymic carcinomas Ki67 was lower in GTF2I mutated (n = 3) tumors compared to WTs (n = 7).
Supplementary Figure 11 Differential expression of isoforms and clustering of transcriptome data.
(a) Cufflinks FPKM values were calculated for each isoform of GTF2I and their average value were reported for wild type (WT: 18 samples) and mutated (MUT: 7 samples) cases. The differential expression was compared using a non-parametric test (Kruskal-Wallis) and the Dunn’s post hoc test that demonstrated significant differences between δ-isoforms compared with α, γ and isoform 5. Similar results were observed for β-isoform. (b) Cufflinks FPKM values were used to cluster TETs with their gene expression data. The clusters trend to segregate TETs according to their histotype. CGH cluster (CGH1 in pink and CGH2 in light blue) and GTF2I mutation status (MUT: Mutant in green and WT: wild type in yellow) also parallel the expression clusters. The two different platforms used to define the profile of gene expression (Genome Analyzer-II (GA-II) and HiSeq2000 (HiSeq) provided equal distribution among expression clusters.
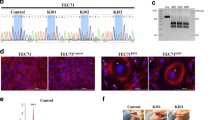
Supplementary Figure 12 Soft agar assay.
(a) Average number of colonies of NIH-3T3 cells transfected with negative control (mock-construct), positive control (HRASV12G), TFII-I β-isoform WT and mutated (p.Leu404His) and δ-isoform of TFII-I WT and mutated (p.Leu383His). For positive and negative controls, results are the average of three experiments. For β- and δ-isoforms, results are the average of three experiments derived from 4 different pool transfectants. Vertical bars represent the standard deviation of triplicate experiments. (b) Pictures of soft agar colonies (5x magnification).
Supplementary Figure 13 FOS expression, TFII-I protein stability and its expression in type A thymomas.
(a) Protein synthesis was inhibited using cycloheximide and cells were harvested at the indicated time points. Proteins were extracted and the amount of TFII-I evaluated by western blot. HELA cells transfected with mutated β-isoform had a more stable TFII-I than those transfected with the WT β-isoform. Similar results were observed with mutated and WT δ-isoforms. (b) Representative TFII-I immunohistochemistry images. Immunohistochemistry performed using anti-TFII-I antibody (not specific for β- or δ- isoform) demonstrated a higher expression in mutated type A thymomas (n = 11) than WTs (n = 4). The pattern of expression was predominantly nuclear.
Supplementary Figure 14 Summary of fusion genes identified by transcriptome sequencing and confirmed by RT-PCR.
The detected fusion genes were reported using Circos. Different colors indicate fusion genes of different TET patients. Details of the identified fusions are reported in Supplementary Table 13.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Figures 1–14 and Supplementary Tables 1, 7, 10 and 11. (PDF 6190 kb)
Supplementary Tables
Supplementary Tables 2–6, 8, 9, and 12–16. (XLSX 10488 kb)
Source data
Rights and permissions
About this article
Cite this article
Petrini, I., Meltzer, P., Kim, IK. et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 46, 844–849 (2014). https://doi.org/10.1038/ng.3016
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3016
This article is cited by
-
Dexamethasone improves thymoma-associated myasthenia gravis via the AKT-mTOR pathway
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
A clinicopathologic study of malignancy in VCP-associated multisystem proteinopathy
Orphanet Journal of Rare Diseases (2022)
-
Thymic epithelial tumors: examining the GTF2I mutation and developing a novel prognostic signature with LncRNA pairs to predict tumor recurrence
BMC Genomics (2022)
-
Human thymoma-associated mutation of the GTF2I transcription factor impairs thymic epithelial progenitor differentiation in mice
Communications Biology (2022)
-
Pan-cancer analysis of necroptosis-related gene signature for the identification of prognosis and immune significance
Discover Oncology (2022)