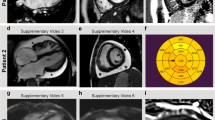
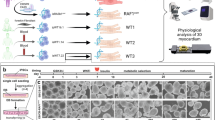
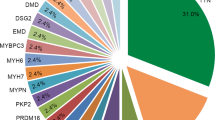
Abstract
Dilated cardiomyopathy (DCM) is a highly heterogeneous trait with sarcomeric gene mutations predominating. The cause of a substantial percentage of DCMs remains unknown, and no gene-specific therapy is available. On the basis of resequencing of 513 DCM cases and 1,150 matched controls from various cohorts of distinct ancestry, we discovered rare, functional RAF1 mutations in 3 of the cohorts (South Indian, North Indian and Japanese). The prevalence of RAF1 mutations was ∼9% in childhood-onset DCM cases in these three cohorts. Biochemical studies showed that DCM-associated RAF1 mutants had altered kinase activity, resulting in largely unaltered ERK activation but in AKT that was hyperactivated in a BRAF-dependent manner. Constitutive expression of these mutants in zebrafish embryos resulted in a heart failure phenotype with AKT hyperactivation that was rescued by treatment with rapamycin. These findings provide new mechanistic insights and potential therapeutic targets for RAF1-associated DCM and further expand the clinical spectrum of RAF1-related human disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Hershberger, R.E., Hedges, D.J. & Morales, A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 10, 531–547 (2013).
Dellefave, L. & McNally, E.M. The genetics of dilated cardiomyopathy. Curr. Opin. Cardiol. 25, 198–204 (2010).
Watkins, H. Genetic clues to disease pathways in hypertrophic and dilated cardiomyopathies. Circulation 107, 1344–1346 (2003).
Watkins, H., Ashrafian, H. & Redwood, C. Inherited cardiomyopathies. N. Engl. J. Med. 364, 1643–1656 (2011).
Herman, D.S. et al. Truncations of titin causing dilated cardiomyopathy. N. Engl. J. Med. 366, 619–628 (2012).
Burkett, E.L. & Hershberger, R.E. Clinical and genetic issues in familial dilated cardiomyopathy. J. Am. Coll. Cardiol. 45, 969–981 (2005).
Sala, V. et al. Signaling to cardiac hypertrophy: insights from human and mouse RASopathies. Mol. Med. 18, 938–947 (2012).
Tidyman, W.E. & Rauen, K.A. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev. 19, 230–236 (2009).
Lin, A.E. et al. Clinical, pathological, and molecular analyses of cardiovascular abnormalities in Costello syndrome: a Ras/MAPK pathway syndrome. Am. J. Med. Genet. A. 155A, 486–507 (2011).
Pandit, B. et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat. Genet. 39, 1007–1012 (2007).
Razzaque, M.A. et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat. Genet. 39, 1013–1017 (2007).
Gelb, B.D. & Tartaglia, M. RAS signaling pathway mutations and hypertrophic cardiomyopathy: getting into and out of the thick of it. J. Clin. Invest. 121, 844–847 (2011).
Kaski, J.P. et al. Prevalence of sequence variants in the RAS–mitogen activated protein kinase signaling pathway in pre-adolescent children with hypertrophic cardiomyopathy. Circ Cardiovasc Genet 5, 317–326 (2012).
Wu, X. et al. Increased BRAF heterodimerization is the common pathogenic mechanism for Noonan syndrome–associated RAF1 mutants. Mol. Cell. Biol. 32, 3872–3890 (2012).
Wu, X. et al. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. J. Clin. Invest. 121, 1009–1025 (2011).
Marin, T.M. et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome–associated PTPN11 mutation. J. Clin. Invest. 121, 1026–1043 (2011).
Dhandapany, P.S. et al. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat. Genet. 41, 187–191 (2009).
Kwan, K.M. et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 (2007).
Acknowledgements
The authors thank A. Mir and C. Evan for their technical support in the zebrafish studies, P. Poulikakos (Icahn School of Medicine at Mount Sinai) and M. Baccarini (Max F. Perutz Laboratories, University of Vienna) for the Braf and Raf1 knockout MEFs, and P. Vaideeswar for the initial collection of samples from cardiomyopathy cases. This work was supported in part by grants from the National Heart, Lung, and Blood Institute to B.D.G. (HL071207) and D.L. (HL097357) and by grants from Telethon-Italy to M.T. (GGP10020 and GGP13107). J.J.E. was a research fellow supported by the Sarnoff Cardiovascular Research Foundation. M.A.R. is a postdoctoral fellow of the American Heart Association (Great Rivers Affiliate). K.T. was supported by a Network project grant (CARDIOMED-BSC0122) from the Council of Scientific and Industrial Research (CSIR) of the government of India.
Author information
Authors and Affiliations
Contributions
P.S.D. conceived the project, with input from B.D.G. P.S.D., M.A.R., U.M., S.K., D.S.R., G.L., A.B., M.K., A.R., E.F., M.T., T.Y., M.F., T. Nishizawa, T. Nakanishi, P.G., B.R., R.M. and K.T. collected and sequenced the various cardiomyopathy cases and controls. P.S.D., M.A.R., J.J.E. and I.R. performed the functional studies, with the help of J.S., S.P. and S.M.-N. J.R., R.J.H., D.L., K.C.S., K.T. and B.D.G. provided reagents for the study. P.S.D. drafted the manuscript, with input from B.D.G.
Corresponding author
Ethics declarations
Competing interests
B.D.G. and P.S.D. are named inventors on a patent application filed with the US Patent and Trademark Office involving RAF1 mutations and dilated cardiomyopathy.
Integrated supplementary information
Supplementary Figure 2 DCM-associated mutant RAF1 proteins hyperactivate AKT but not ERK1/2.
Representative immunoblot with total lysates from HEK293 cells expressing wild-type RAF1 (WT), DCM-associated mutant RAF1 proteins (Pro332Ala, Leu603Pro, His626Arg, Thr641Met and Arg254fs) and HCM-associated mutant RAF1 proteins (Ser257Leu and Leu613Val) that were stimulated with EGF for 15 min. The blot was probed with anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-AKT, anti-AKT and anti-RAF1 antibodies. Expression levels were normalized to respective total protein levels and are expressed as relative expression compared to the levels in the cells overexpressing wild-type RAF1. GAPDH levels were used as a loading control. Data are the means ± s.d. from two independent experiments assayed in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001 compared to wild type.
Supplementary Figure 3 DCM-associated RAF1 mutants activate the AKT-mTOR pathway as indicated by tuberin phosphorylation.
Representative immunoblot with total lysates from HEK293 cells expressing wild-type RAF1 (WT), DCM-associated mutant RAF1 proteins (Pro332Ala, Leu603Pro, His626Arg, Thr641Met and Arg254fs) and HCM-associated mutant RAF1 proteins (Ser257Leu and Leu613Val) that were stimulated with EGF for 15 min. The blot was probed with anti-phospho-TSC2, anti-RAF1 and anti-GAPDH antibodies. Expression levels were normalized to RAF1 levels and are expressed as relative expression compared to levels in cells expressing wild-type RAF1. GAPDH levels were used as a loading control. Data are the means ± s.d. from two independent experiments assayed in duplicate. **P < 0.01 compared to wild type.
Supplementary Figure 4 DCM-associated RAF1 mutants show impaired Erk1/2 activation in Raf1–/– MEFs.
Immunoblot with total lysates from MEFs from Raf1 knockout mice (Raf1–/–) expressing wild-type RAF1 (WT) and two representative DCM mutants (Pro332Ala and Leu603Pro) that were stimulated with EGF for 0, 5, 15, 30, 45 and 60 min and probed with anti-phospho-Erk1/2, anti-Erk1/2 and anti-RAF1 antibodies. Expression levels were normalized to respective total protein levels and are expressed as relative expression compared to levels in cells expressing wild-type RAF1 at the indicated time points. β-actin levels were used as a loading control.
Supplementary Figure 5 DCM-associated RAF1 mutants fail to activate Erk1/2 in Braf–/– MEFs.
Immunoblot with total lysates from MEFs from Braf knockout mice (Braf –/–) expressing wild-type RAF1 (WT) and two representative DCM mutants (Pro332Ala and Leu603Pro) that were stimulated with EGF for 0, 5, 15 and 30 min and probed with anti-phospho-Erk1/2, anti-Erk2 and anti-RAF1 antibodies. Expression levels were normalized to respective total protein levels and are expressed as relative expression compared to levels in cells expressing wild-type RAF1 at the indicated time points. β-actin levels were used as a loading control.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 1979 kb)
Rights and permissions
About this article
Cite this article
Dhandapany, P., Razzaque, M., Muthusami, U. et al. RAF1 mutations in childhood-onset dilated cardiomyopathy. Nat Genet 46, 635–639 (2014). https://doi.org/10.1038/ng.2963
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2963
This article is cited by
-
Dilated cardiomyopathy: a new insight into the rare but common cause of heart failure
Heart Failure Reviews (2022)
-
Systematic analysis of the intersection of disease mutations with protein modifications
BMC Medical Genomics (2019)
-
Translating emerging molecular genetic insights into clinical practice in inherited cardiomyopathies
Journal of Molecular Medicine (2018)
-
Clinical relevance of screening checklists for detecting cancer predisposition syndromes in Asian childhood tumours
npj Genomic Medicine (2018)
-
Cardiac Gab1 deletion leads to dilated cardiomyopathy associated with mitochondrial damage and cardiomyocyte apoptosis
Cell Death & Differentiation (2016)