Abstract
Adrenal tumors autonomously producing cortisol cause Cushing's syndrome1,2,3,4. We performed exome sequencing of 25 tumor-normal pairs and identified 2 subgroups. Eight tumors (including three carcinomas) had many somatic copy number variants (CNVs) with frequent deletion of CDC42 and CDKN2A, amplification of 5q31.2 and protein-altering mutations in TP53 and RB1. Seventeen tumors (all adenomas) had no somatic CNVs or TP53 or RB1 mutations. Six of these had known gain-of-function mutations in CTNNB1 (β-catenin)5,6 or GNAS (Gαs)7,8. Six others had somatic mutations in PRKACA (protein kinase A (PKA) catalytic subunit) resulting in a p.Leu206Arg substitution. Further sequencing identified this mutation in 13 of 63 tumors (35% of adenomas with overt Cushing's syndrome). PRKACA, GNAS and CTNNB1 mutations were mutually exclusive. Leu206 directly interacts with the regulatory subunit of PKA, PRKAR1A9,10. Leu206Arg PRKACA loses PRKAR1A binding, increasing the phosphorylation of downstream targets. PKA activity induces cortisol production and cell proliferation11,12,13,14,15, providing a mechanism for tumor development. These findings define distinct mechanisms underlying adrenal cortisol-producing tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Referenced accessions
NCBI Reference Sequence
Protein Data Bank
Change history
05 June 2014
In the version of this article initially published, the name of author John W. Kunstman was misspelled. The error has been corrected in the HTML and PDF versions of the article.
References
Hatipoglu, B.A. Cushing's syndrome. J. Surg. Oncol. 106, 565–571 (2012).
Orth, D.N. Cushing's syndrome. N. Engl. J. Med. 332, 791–803 (1995).
Biller, B.M. et al. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J. Clin. Endocrinol. Metab. 93, 2454–2462 (2008).
Chiodini, I. Clinical review: diagnosis and treatment of subclinical hypercortisolism. J. Clin. Endocrinol. Metab. 96, 1223–1236 (2011).
El Wakil, A. & Lalli, E. The Wnt/β-catenin pathway in adrenocortical development and cancer. Mol. Cell. Endocrinol. 332, 32–37 (2011).
Tissier, F. et al. Mutations of β-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 65, 7622–7627 (2005).
Almeida, M.Q. & Stratakis, C.A. How does cAMP/protein kinase A signaling lead to tumors in the adrenal cortex and other tissues? Mol. Cell. Endocrinol. 336, 162–168 (2011).
Fragoso, M.C. et al. Cushing's syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J. Clin. Endocrinol. Metab. 88, 2147–2151 (2003).
Kim, C., Xuong, N.H. & Taylor, S.S. Crystal structure of a complex between the catalytic and regulatory (RIα) subunits of PKA. Science 307, 690–696 (2005).
Ubersax, J.A. & Ferrell, J.E. Jr. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 8, 530–541 (2007).
Bertherat, J. et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 63, 5308–5319 (2003).
Casey, M. et al. Mutations in the protein kinase A R1α regulatory subunit cause familial cardiac myxomas and Carney complex. J. Clin. Invest. 106, R31–R38 (2000).
Cazabat, L., Ragazzon, B., Groussin, L. & Bertherat, J. PRKAR1A mutations in primary pigmented nodular adrenocortical disease. Pituitary 9, 211–219 (2006).
Cho-Chung, Y.S., Pepe, S., Clair, T., Budillon, A. & Nesterova, M. cAMP-dependent protein kinase: role in normal and malignant growth. Crit. Rev. Oncol. Hematol. 21, 33–61 (1995).
Kirschner, L.S. et al. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the Carney complex. Hum. Mol. Genet. 9, 3037–3046 (2000).
Saffran, M. Mechanisms of adrenocortical control. Br. Med. Bull. 18, 122–126 (1962).
Stocco, D.M. & Clark, B.J. Regulation of the acute production of steroids in steroidogenic cells. Endocr. Rev. 17, 221–244 (1996).
Gillies, G.E., Linton, E.A. & Lowry, P.J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 299, 355–357 (1982).
Rivier, C. & Vale, W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature 305, 325–327 (1983).
Ramachandran, J., Tsubokawa, M. & Gohil, K. Corticotropin receptors. Ann. NY Acad. Sci. 512, 415–425 (1987).
Simpson, E.R. & Waterman, M.R. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu. Rev. Physiol. 50, 427–440 (1988).
Roger, P.P., Reuse, S., Maenhaut, C. & Dumont, J.E. Multiple facets of the modulation of growth by cAMP. Vitam. Horm. 51, 59–191 (1995).
Rosenberg, D. et al. Role of the PKA-regulated transcription factor CREB in development and tumorigenesis of endocrine tissues. Ann. NY Acad. Sci. 968, 65–74 (2002).
Newell-Price, J., Trainer, P., Besser, M. & Grossman, A. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr. Rev. 19, 647–672 (1998).
Almeida, M.Q. & Stratakis, C.A. Carney complex and other conditions associated with micronodular adrenal hyperplasias. Best Pract. Res. Clin. Endocrinol. Metab. 24, 907–914 (2010).
Horvath, A. et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat. Genet. 38, 794–800 (2006).
Horvath, A. et al. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res. 66, 11571–11575 (2006).
Nieman, L.K. et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 93, 1526–1540 (2008).
Mermel, C.H. et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12, R41 (2011).
Tömböl, Z. et al. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocr. Relat. Cancer 16, 895–906 (2009).
Bonnet, S. et al. Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J. Clin. Endocrinol. Metab. 96, E419–E426 (2011).
Zhu, J. et al. The UCSC Cancer Genomics Browser. Nat. Methods 6, 239–240 (2009).
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012).
Hsiao, H.P. et al. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J. Clin. Endocrinol. Metab. 94, 2930–2937 (2009).
Songyang, Z. et al. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol. 4, 973–982 (1994).
Taylor, S.S. et al. Dynamics of signaling by PKA. Biochim. Biophys. Acta 1754, 25–37 (2005).
Zheng, J. et al. 2.2 A refined crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MnATP and a peptide inhibitor. Acta Crystallogr. D Biol. Crystallogr. 49, 362–365 (1993).
de Joussineau, C. et al. The cAMP pathway and the control of adrenocortical development and growth. Mol. Cell. Endocrinol. 351, 28–36 (2012).
Lania, A.G., Mantovani, G. & Spada, A. Mechanisms of disease: mutations of G proteins and G-protein-coupled receptors in endocrine diseases. Nat. Clin. Pract. Endocrinol. Metab. 2, 681–693 (2006).
Meoli, E. et al. Protein kinase A effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res. 68, 3133–3141 (2008).
Choi, M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331, 768–772 (2011).
Scholl, U.I. et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 45, 1050–1054 (2013).
Scholl, U.I. et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc. Natl. Acad. Sci. USA 109, 2533–2538 (2012).
Almeida, M.Q. et al. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/− or Rb1+/− backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum. Mol. Genet. 19, 1387–1398 (2010).
Gaujoux, S. et al. Wnt/β-catenin and 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A signaling pathways alterations and somatic β-catenin gene mutations in the progression of adrenocortical tumors. J. Clin. Endocrinol. Metab. 93, 4135–4140 (2008).
Hino, S., Tanji, C., Nakayama, K.I. & Kikuchi, A. Phosphorylation of β-catenin by cyclic AMP–dependent protein kinase stabilizes β-catenin through inhibition of its ubiquitination. Mol. Cell. Biol. 25, 9063–9072 (2005).
Taurin, S., Sandbo, N., Qin, Y., Browning, D. & Dulin, N.O. Phosphorylation of β-catenin by cyclic AMP–dependent protein kinase. J. Biol. Chem. 281, 9971–9976 (2006).
Schinner, S. et al. Adipocyte-derived products induce the transcription of the StAR promoter and stimulate aldosterone and cortisol secretion from adrenocortical cells through the Wnt-signaling pathway. Int. J. Obes. (Lond.) 31, 864–870 (2007).
Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 497, 67–73 (2013).
Cleary, S.P. et al. Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology 58, 1693–1702 (2013).
Beuschlein, F. et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing's syndrome. N. Engl. J. Med. 370, 1019–1028 (2014).
Zenkert, S. et al. Steroidogenic acute regulatory protein mRNA expression in adrenal tumours. Eur. J. Endocrinol. 142, 294–299 (2000).
Brauckhoff, M. et al. Peritoneal carcinosis in apparently benign cortisol producing adrenal adenoma ≥ 5 cm in diameter: the need of regular postoperative surveillance. Exp. Clin. Endocrinol. Diabetes 120, 472–476 (2012).
Almeida, M.Q. et al. Integrated genomic analysis of nodular tissue in macronodular adrenocortical hyperplasia: progression of tumorigenesis in a disorder associated with multiple benign lesions. J. Clin. Endocrinol. Metab. 96, E728–E738 (2011).
Heaton, J.H. et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am. J. Pathol. 181, 1017–1033 (2012).
Ronchi, C.L. et al. Single nucleotide polymorphism array profiling of adrenocortical tumors—evidence for an adenoma carcinoma sequence? PLoS ONE 8, e73959 (2013).
Dhanasekaran, N., Heasley, L.E. & Johnson, G.L. G protein–coupled receptor systems involved in cell growth and oncogenesis. Endocr. Rev. 16, 259–270 (1995).
Lau, S.K. & Weiss, L.M. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum. Pathol. 40, 757–768 (2009).
Duregon, E. et al. Oncocytic adrenocortical tumors: diagnostic algorithm and mitochondrial DNA profile in 27 cases. Am. J. Surg. Pathol. 35, 1882–1893 (2011).
Lawrence, M.S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Farrell, C.M. et al. Current status and new features of the Consensus Coding Sequence database. Nucleic Acids Res. 42, D865–D872 (2014).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Trapnell, C. et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31, 46–53 (2013).
Shibata, S., Zhang, J., Puthumana, J., Stone, K.L. & Lifton, R.P. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc. Natl. Acad. Sci. USA 110, 7838–7843 (2013).
Acknowledgements
This work was supported in part by the US National Institutes of Health (NIH) Centers for Mendelian Genomics (5U54HG006504). G.G. is supported by the Agency for Science, Technology and Research of Singapore. T.C. is a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation. R.P.L. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
U.I.S., J.M.H., M.L.P., J.W.K., R.K., A.-C.S., D.D., M.H., H.S.W., P.S., P.H., P.B., G.Å. and T.C. ascertained and recruited subjects and obtained samples and medical records. U.I.S., R.K., P.B. and C.N.-W. prepared DNA and RNA samples and maintained sample archives. G.G. performed and analyzed targeted DNA and RNA sequencing. G.G., M.C. and R.P.L. analyzed exome sequencing and RNA sequencing results. G.G. generated constructs and performed immunoprecipitation and protein blot analysis. G.G. and J.M.H. performed immunohistochemistry. G.G. and M.L.P. reviewed histology and immunohistochemistry. G.G., U.I.S. and R.P.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Overview of copy number variants in CNV-positive tumors.
Frequency of gains (red) and losses (blue) plotted across genome.
Supplementary Figure 2 Significant, focal CNVs in tumors.
Left, deletions in blue. Right, amplifications in red. The green line signifies the threshold for significance, q = 0.25.
Supplementary Figure 3 RNA-seq of a PRKACA-mutant tumor.
Both wild-type (A) and mutant (C) alleles are detected in the tumor, as shown in the position highlighted.
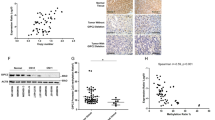
Supplementary Figure 4 Increased levels of phosphorylated CREB in PRKACA-mutant tumors.
Representative images of PRKACA-mutant tumor and wild-type tumor (no mutation in PRKACA, CTNNB1 or GNAS) stained for CREB phosphorylated at Ser133. Intense nuclear-specific staining is observed in the majority of the nuclei in the mutant tumor (a) but not in the wild-type tumor (b). Scale bars represent 60 μm.
Supplementary Figure 5 Histology of PRKACA-mutant tumors.
All tumors reviewed with PRKACA mutations (n = 6) were well-defined, solitary adrenal cortical nodules with well-differentiated morphology. The majority of these tumors had low-grade nuclei (5/6), and none of the tumors showed fibrosis, necrosis, mitosis and hemorrhage, consistent with benign adenomas. (a) 2.6-cm cortisol-secreting adrenal cortical adenoma showing a bright yellow and brown cut surface that corresponds to its cellular composition as shown in b. The ruler is in centimeters and millimeters. (b) The tumor comprises lipid-rich clear cells (filled arrow) and oncocytic cells (open arrow) with granular eosinophilic cytoplasm. The scale bar represents 60 μm.
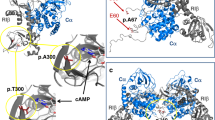
Supplementary Figure 6 Model for cortisol-producing adrenal tumor formation due to gain-of-function mutation in PRKACA.
(a) In quiescent adrenal fasciculata cells, protein kinase A exists as an inactive tetramer, with the catalytic subunits bound to a dimer of regulatory subunits. (b) ACTH binds to its receptor, melanocortin receptor 2, which stimulates adenylyl cyclase via Gas, leading to an increase in intracellular cAMP levels. cAMP binds to the regulatory subunits, causing the release of the catalytic subunits and downstream signaling, which leads to cell proliferation and cortisol production. (c) The mutant catalytic subunits are not bound by the regulatory subunit and are thus constitutively active in the absence of external stimuli. The resulting uncontrolled cell proliferation and cortisol production lead to the formation of a cortisol-producing adrenal tumor. MC2, melanocortin receptor 2; AC, adenylyl cyclase; R, regulatory subunit; C, catalytic subunit.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–7 and Supplementary Figures 1–6 (PDF 5510 kb)
Rights and permissions
About this article
Cite this article
Goh, G., Scholl, U., Healy, J. et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat Genet 46, 613–617 (2014). https://doi.org/10.1038/ng.2956
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2956
This article is cited by
-
A machine learning approach to distinguishing between non-functioning and autonomous cortisol secreting adrenal incidentaloma on magnetic resonance imaging using texture analysis
Irish Journal of Medical Science (1971 -) (2023)
-
The effects of 20-hydroxyecdysone on nuclear shape, heterochromatin quantity and gray-level co-occurrence matrix texture analysis of adrenal zona fasciculata cells in an obese gerbil (Gerbillus tarabuli) model for metabolic syndrome: a correlational study
Histochemistry and Cell Biology (2023)
-
Oncogene addiction to GNAS in GNASR201 mutant tumors
Oncogene (2022)
-
Overview of the 2022 WHO Classification of Adrenal Cortical Tumors
Endocrine Pathology (2022)
-
Clinical analysis of the etiological spectrum of bilateral adrenal lesions: A large retrospective, single-center study
Endocrine (2022)