Abstract
Down syndrome confers a 20-fold increased risk of B cell acute lymphoblastic leukemia (B-ALL)1, and polysomy 21 is the most frequent somatic aneuploidy among all B-ALLs2. Yet the mechanistic links between chromosome 21 triplication and B-ALL remain undefined. Here we show that germline triplication of only 31 genes orthologous to human chromosome 21q22 confers mouse progenitor B cell self renewal in vitro, maturation defects in vivo and B-ALL with either the BCR-ABL fusion protein or CRLF2 with activated JAK2. Chromosome 21q22 triplication suppresses histone H3 Lys27 trimethylation (H3K27me3) in progenitor B cells and B-ALLs, and 'bivalent' genes with both H3K27me3 and H3K4me3 at their promoters in wild-type progenitor B cells are preferentially overexpressed in triplicated cells. Human B-ALLs with polysomy 21 are distinguished by their overexpression of genes marked with H3K27me3 in multiple cell types. Overexpression of HMGN1, a nucleosome remodeling protein encoded on chromosome 21q22 (refs. 3,4,5), suppresses H3K27me3 and promotes both B cell proliferation in vitro and B-ALL in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rabin, K.R. & Whitlock, J.A. Malignancy in children with trisomy 21. Oncologist 14, 164–173 (2009).
Heerema, N.A. et al. Specific extra chromosomes occur in a modal number dependent pattern in pediatric acute lymphoblastic leukemia. Genes Chromosom. Cancer 46, 684–693 (2007).
Catez, F., Brown, D.T., Misteli, T. & Bustin, M. Competition between histone H1 and HMGN proteins for chromatin binding sites. EMBO Rep. 3, 760–766 (2002).
Lim, J.H. et al. Chromosomal protein HMGN1 enhances the acetylation of lysine 14 in histone H3. EMBO J. 24, 3038–3048 (2005).
Rattner, B.P., Yusufzai, T. & Kadonaga, J.T. HMGN proteins act in opposition to ATP-dependent chromatin remodeling factors to restrict nucleosome mobility. Mol. Cell 34, 620–626 (2009).
Olson, L.E., Richtsmeier, J.T., Leszl, J. & Reeves, R.H. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science 306, 687–690 (2004).
Moorman, A.V. et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 11, 429–438 (2010).
Hardy, R.R., Carmack, C.E., Shinton, S.A., Kemp, J.D. & Hayakawa, K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173, 1213–1225 (1991).
Reeves, R.H. et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat. Genet. 11, 177–184 (1995).
Roy, A. et al. Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc. Natl. Acad. Sci. USA 109, 17579–17584 (2012).
Mullighan, C.G. et al. Rearrangement of CRLF2 in B-progenitor– and Down syndrome–associated acute lymphoblastic leukemia. Nat. Genet. 41, 1243–1246 (2009).
Russell, L.J. et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood 114, 2688–2698 (2009).
Yoda, A. et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 107, 252–257 (2010).
Iacobucci, I. et al. Expression of spliced oncogenic Ikaros isoforms in Philadelphia-positive acute lymphoblastic leukemia patients treated with tyrosine kinase inhibitors: implications for a new mechanism of resistance. Blood 112, 3847–3855 (2008).
Mullighan, C.G. et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 106, 9414–9418 (2009).
Wetzler, M. et al. Additional cytogenetic abnormalities in adults with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a study of the Cancer and Leukaemia Group B. Br. J. Haematol. 124, 275–288 (2004).
Krause, D.S., Lazarides, K., von Andrian, U.H. & Van Etten, R.A. Requirement for CD44 in homing and engraftment of BCR-ABL–expressing leukemic stem cells. Nat. Med. 12, 1175–1180 (2006).
Cabelof, D.C. et al. Mutational spectrum at GATA1 provides insights into mutagenesis and leukemogenesis in Down syndrome. Blood 114, 2753–2763 (2009).
Hertzberg, L. et al. Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: a report from the International BFM Study Group. Blood 115, 1006–1017 (2010).
Smith, J.R. et al. Robust, persistent transgene expression in human embryonic stem cells is achieved with AAVS1-targeted integration. Stem Cells 26, 496–504 (2008).
Weinstock, D.M. & Jasin, M. Alternative pathways for the repair of RAG-induced DNA breaks. Mol. Cell. Biol. 26, 131–139 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Merico, D., Isserlin, R., Stueker, O., Emili, A. & Bader, G.D. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE 5, e13984 (2010).
Mikkelsen, T.S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Ben-Porath, I. et al. An embryonic stem cell–like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 40, 499–507 (2008).
Bernstein, B.E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Lin, Y.C. et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat. lmmunol. 11, 635–643 (2010).
Kruidenier, L. et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 488, 404–408 (2012).
McCabe, M.T. et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492, 108–112 (2012).
Rochman, M. et al. Effects of HMGN variants on the cellular transcription profile. Nucleic Acids Res. 39, 4076–4087 (2011).
Bustin, M. et al. Characterization of transgenic mice with an increased content of chromosomal protein HMG-14 in their chromatin. DNA Cell Biol. 14, 997–1005 (1995).
Urbánek, P., Wang, Z.Q., Fetka, I., Wagner, E.F. & Busslinger, M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 79, 901–912 (1994).
Dildrop, R. et al. IgH enhancer-mediated deregulation of N-myc gene expression in transgenic mice: generation of lymphoid neoplasias that lack c-myc expression. EMBO J. 8, 1121–1128 (1989).
Abuhatzira, L., Shamir, A., Schones, D.E., Schaffer, A.A. & Bustin, M. The chromatin-binding protein HMGN1 regulates the expression of methyl CpG-binding protein 2 (MECP2) and affects the behavior of mice. J. Biol. Chem. 286, 42051–42062 (2011).
Birger, Y. et al. Chromosomal protein HMGN1 enhances the rate of DNA repair in chromatin. EMBO J. 22, 1665–1675 (2003).
Bustin, M., Crippa, M.P. & Pash, J.M. Immunochemical analysis of the exposure of high mobility group protein 14 and 17 surfaces in chromatin. J. Biol. Chem. 265, 20077–20080 (1990).
Trageser, D. et al. Pre-B cell receptor–mediated cell cycle arrest in Philadelphia chromosome–positive acute lymphoblastic leukemia requires IKAROS function. J. Exp. Med. 206, 1739–1753 (2009).
Tomizuka, K. et al. Functional expression and germline transmission of a human chromosome fragment in chimaeric mice. Nat. Genet. 16, 133–143 (1997).
Fung, H. & Weinstock, D.M. Repair at single targeted DNA double-strand breaks in pluripotent and differentiated human cells. PLoS ONE 6, e20514 (2011).
Weinstock, D.M., Nakanishi, K., Helgadottir, H.R. & Jasin, M. Assaying double-strand break repair pathway choice in mammalian cells using a targeted endonuclease or the RAG recombinase. Methods Enzymol. 409, 524–540 (2006).
Ashton, J.M. et al. Gene sets identified with oncogene cooperativity analysis regulate in vivo growth and survival of leukemia stem cells. Cell Stem Cell 11, 359–372 (2012).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Robinson, M.D., McCarthy, D.J. & Smyth, G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Robinson, M.D. & Smyth, G.K. Moderated statistical tests for assessing differences in tag abundance. Bioinformatics 23, 2881–2887 (2007).
Robinson, M.D. & Smyth, G.K. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 9, 321–332 (2008).
Irizarry, R.A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003).
Dai, M. et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 33, e175 (2005).
Reich, M. et al. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
Kinsella, R.J. et al. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database (Oxford) 2011, bar030 (2011).
Peach, S.E., Rudomin, E.L., Udeshi, N.D., Carr, S.A. & Jaffe, J.D. Quantitative assessment of chromatin immunoprecipitation grade antibodies directed against histone modifications reveals patterns of co-occurring marks on histone protein molecules. Molecular & cellular proteomics. Mol. Cell. Proteomics 11, 128–137 (2012).
Verzi, M.P. et al. Differentiation-specific histone modifications reveal dynamic chromatin interactions and partners for the intestinal transcription factor CDX2. Dev. Cell 19, 713–726 (2010).
Lin, C.Y. et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151, 56–67 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Shin, H., Liu, T., Manrai, A.K. & Liu, X.S. CEAS: cis-regulatory element annotation system. Bioinformatics 25, 2605–2606 (2009).
Lovén, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Acknowledgements
We thank N. Kopp and A. Schlauch for technical assistance, M. Busslinger (Research Institute of Molecular Pathology, Vienna) for the Pax5+/− mice and M. Oshimura (Tottori University) for A9 cells carrying human chromosome 21. This research was supported by the Conquer Cancer Foundation (A.A.L.), the Lauri Strauss Leukemia Foundation (A.A.L.), the Leukemia and Lymphoma Society (A.A.L.), the Alex Lemonade Stand Foundation (A.A.L., H. Li and D.P.), the US Department of Defense (C.Y.L.), the Israel Science Foundation (S.I.), the US Israel Binational Foundation (J.D.C. and S.I.), the Stellato Fund (D.M.W.), US National Institutes of Health/National Cancer Institute R01 awards CA15198-01 and CA172387-A01 (D.M.W.) and a Translational Research Award from the Leukemia and Lymphoma Society (J.D.C. and D.M.W.).
Author information
Authors and Affiliations
Contributions
A.A.L., T.T., H. Li, E.C.T., D.v.B., T.A.D., S.-C.W., H. Liu, A.Y., S.M., J.E.T., J.D.J. and G.t.K. designed and performed experiments. A.A.L., B.C., C.Y.L., G.A., T.J.S., K.S., K.E.S., D.N., J.D.J., S.I., J.D.C., D.P. and D.M.W. analyzed data. A.C.S., J.E.B., W.C.H. and J.D.J. developed analytical tools. M.B., M.H.H., L.B.S., S.E.S. and D.P. provided essential reagents. A.A.L. and D.M.W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
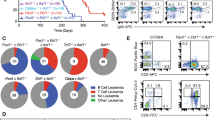
Supplementary Figure 1 Abnormal differentiation in vivo and colony growth in vitro of B cells with triplication of chromosome 21 orthologs.
(a) (Top) B220 and CD43 staining of bone marrow from Ts1Rhr and wild-type mice, highlighting the more immature B220+CD43+ and more mature B220+CD43- B cell populations. (Bottom) CD24 and BP1 staining of the B220+CD43+ subpopulation demonstrates the early Hardy fractions: A (CD24- BP1-), B (CD24+BP1-), and C (CD24+BP1+). (b) Hardy subfractions of the B220+CD43+ population as absolute percentages of bone marrow mononuclear cells by flow cytometry from Ts65Dn (blue) or C57BL/6 Ts1Rhr (orange) animals compared to wild-type littermate (black) mice (n=4 mice per genotype). (c) Schematic for the competitive bone marrow transplantation assay. (d) Representative Hardy fraction staining in bone marrow gated on CD45.2 negative (left) competitor cells or CD45.2 positive (right) test cells. The top rows are wild-type test cells, and the bottom rows are Ts1Rhr test cells. There are fewer Ts1Rhr Hardy B/C cells and greater numbers of Ts1Rhr Hardy A cells in recipients of wild-type:Ts1Rhr competitive transplants (bottom right). (e) Schematic of the methylcelluose replating assay. Whole BM from Ts1Rhr or wild-type mice was plated in semi-solid medium containing cytokines favoring B cell or myeloid colony growth. 50,000 cells were collected from pooled colonies every seven days and replated in fresh media. (f) Cell surface phenotype of passage 1 B cell colonies from Ts1Rhr and wild-type animals is similar. Representative flow cytometry plots of Hardy fraction cell surface phenotype of passage 1 Ts1Rhr and wild-type B cell colonies is shown. All cells are also B220+CD43+ (not shown).
Supplementary Figure 2 Characterization of the B-ALL that arises in Ts1Rhr bone marrow.
(a) Representative phenotype of C2/J2/P5/Ts1 + Ik6 B-ALL is shown, demonstrating expression of human CRLF2 in the leukemic B cells that also co-express dominant negative Ikaros (Ik6). (b) Leukemia-free survival for wild-type mice after transplantation with bone marrow of the genotypes listed transduced with dominant negative Ikaros (Ik6) (n=6-8 mice/group, **P<0.01 for C2/J2/P5 + Ik6 versus any other genotype by log-rank test). (c) Transduced Ts1Rhr and wild-type bone marrow is shown using flow cytometry for B220 and GFP (BCR-ABL) demonstrating approximately equal proportions of GFP+ cells at the time of transplantation. (d) Ts1Rhr and wild-type BCR-ABL B-ALLs demonstrate similar splenomegaly at the time of death with leukemia. Red dotted line represents upper limit of normal spleen weight. (e) Bone marrow and spleen histology by hematoxylin and eosin staining shows similar infiltration with B-ALL cells in Ts1Rhr and wild-type B-ALLs (scale bar = 50 μm). (f) Survival curves for recipients of Ts1Rhr or wild-type bone marrow cells (on a C57BL/6 background) transduced with BCR-ABL (n=9 mice per group, curves compared by log-rank test). (g) Increase in B-ALL from Ts1Rhr bone marrow is progenitor B cell autonomous. Hardy B cells were sorted from Ts1Rhr or wild-type bone marrow, transduced with BCR-ABL, and equal numbers of cells were transplanted into wild-type recipients. (n=5 mice per group, curves compared by log-rank test).
Supplementary Figure 3 Trisomy and tetrasomy 21 retinal pigment epithelium (RPE) cells generated by microcell-mediated chromosome transfer (MMCT) do not have differences in DNA repair after I-SceI or RAG-induced cleavage.
(a) Single nucleotide polymorphism (SNP) array data for a tetrasomy 21 RPE clone (tetra 21-1), two trisomy 21 (tri21-2 and tri21-3) clones, and a diploid clone are shown across the entire genome (top) or chromosome 21 (bottom). (b) Representative fluorescence in situ hybridization for human chr.21 in trisomy 21 and tetrasomy 21 RPE cells (red = chr.21 probe, blue = DAPI). (c) Representative G-banding karyotype for a tetrasomy 21 RPE cell line. (d) The DR-GFP construct was targeted to the p84 locus in RPE cells containing 2 or more copies of chr.21. A single double-strand DNA break induced by I-SceI can be repaired by multiple pathways. (e) Repair after I-SceI cleavage in cells lacking classical nonhomologous end-joining (NHEJ) factors (e.g. KU70/80, XRCC4/LIG4) is characterized by higher rates of homologous recombination and more extensive deletions at NHEJ junctions (Pierce et al. Genes Dev 15:3237-42). However, the frequencies of homologous recombination (shown as percent GFP-positive) induced by I-SceI do not significantly differ between disomic (Di) and trisomy 21 (Tri) RPE clones. Two clones from each genotype were assayed on two occasions in triplicate. (f) Similarly, the phenotype of nonhomologous end-joining induced by I-SceI did not significantly differ between disomic and trisomy 21 RPE clones. The number of base pairs deleted at junctions formed by NHEJ from two clones from each genotype is shown. (g) The DR-GFP-CE construct targeted to the p84 locus can be used to assess repair after RAG cleavage. Cleavage at the paired RAG recognition signal sequences (white and black triangles) results in removal of the intervening sequence (in yellow) and nonhomologous end joining (NHEJ) between the double-strand break ends. (h) PCR shows no difference in the frequencies of the RAG-induced deletion between diploid and tetrasomy 21 cells. Two biologic replicates are shown for each genotype. (i) Repair junctions after RAG cleavage in cells lacking classical NHEJ factors (e.g. KU70/80, XRCC4/LIG4) typically have longer deletions and more extensive use of short stretches of homology than in wild-type cells (Weinstock et al. Mol Cell Biol 26:131-9). However, the number of base pairs deleted after cleavage by RAG and NHEJ did not significantly differ between disomic and tetrasomy 21 cells (n=2 clones per genotype). (j) The junction sequences are shown for disomic (n=27) and tetrasomy 21 (n=70) RPE clones. A single nucleotide insertion is shown in Tetra-1 # B-3-7 (yellow).
Supplementary Figure 4 RNA-seq expression of the triplicated genes in Ts1Rhr compared to wild-type B cells.
RNA sequencing of Ts1Rhr and wild-type B cells (n=3 mice per genotype) showing relative expression levels among the 25 expressed triplicated genes (absolute fragments per kilobase per million reads [FPKM] > 0.1), and the flanking centromeric and telomeric regions.
Supplementary Figure 5 Down syndrome–associated ALL is associated with overexpression of PRC2 targets, genes marked by H3K27me3, Ts1Rhr and PRC2/H3K27me3 gene signatures distinguish non–Down syndrome–associated ALL with somatic gain of chromosome 21 or iAMP21, and Ts1Rhr B-ALLs are associated with H3K27 hypomethylation.
(a) Heat maps of all genes comprising three of the top five scoring target gene sets enriched in the core Ts1Rhr signature in Down syndrome-ALLs and non-Down syndrome-ALLs. (b) Unsupervised clustering of a validation cohort of 30 non-Down syndrome pediatric B-ALL gene expression signatures (the AIEOP-2 cohort) is shown using a 100-gene SUZ12 target gene set. Four patients with somatic gain of chr.21 and two with iAMP21 cluster within a distinct group with 5 additional cases (P=0.001 by Fisher's exact test). (c) GSEA plots of the Ts1Rhr gene set and the top 100 discriminating genes in the Mikkelsen NPC and MEF H3K27me3 gene sets from the AIEOP cohort, queried in the primary human B-ALLs in the AIEOP-2 cohort containing cases with somatic +21 and iAMP21. ES indicates enrichment score. (d) Unsupervised hierarchical clustering of histone H3 post-translational modifications in splenocytes from mice with Ts1Rhr and wild-type BCR-ABL B-ALLs quantitated by mass spectrometry (blue-red = low-to-high relative amount of each listed peptide, n=3 independent leukemias for each genotype). Peptides containing H3K27me3 with lower abundance in Ts1Rhr B-ALLs are indicated by arrows. (e) Western blotting in sorted CD19+ Ts1Rhr and wild-type B-ALLs (n=5 independent leukemias for each genotype, distinct from those in panel D).
Supplementary Figure 6 ChIP-seq and CHIP-qPCR show decreased H3K27me3 at promoters in Ts1Rhr B cells, the Ts1Rhr gene set is enriched for E2A/TCF3 and LEF1 targets, and Down syndrome–associated ALLs are sensitive to GSK-J4.
(a) ChIP for H3K27me3 (left), H3K27me3 (right), or control rabbit IgG followed by quantitative PCR on a representative set of genes from the Ts1Rhr signature in an independent validation set of wild-type and Ts1Rhr mice (n=3 mice per genotype, one representative of two independent experiments). Data represented as fold enrichment over input relative to a negative control intergenic region on chr.5 (Chr 5 IN) (**P<0.01, *P<0.05). (b) H3K27me3 enriched regions in wild-type B cells. The promoter region is defined as the 5kb flanking annotated transcription start sites. Overlap of H3K27me3 regions with the promoter region was significant in comparison to a random background model of the genome (P<10-10). (c) Venn diagram showing the number and overlap between H3K27me3 enriched regions in wild-type (WT) or Ts1Rhr B cells. (d) The log2 fold difference in density of H3K27me3 at promoters between Ts1Rhr and wild-type B cells is shown. (e) Top three ranked transcription factors with predicted binding sites among promoters of genes in the listed sets as queried in MSigDB “c3.tft” defined in the TRANSFAC database (version 7.4, www.gene-regulation.com). (f) Relative fraction of genes that have proximal E2A/TCF3 occupancy among all genes (7129 of 20671), genes with only H3K27me3 (557 of 1994) or H3K4me3 (4032 of 9360) at the promoter in wild-type B cells, or genes in the Ts1Rhr gene set (85 of 150) (**P<0.01, ***P<0.0001 versus the Ts1Rhr gene set by Chi-square with Yates' correction). (g) Expression of genes in the Ts1Rhr and Core Ts1Rhr sets are increased compared to all probesets in wild-type B cell progenitors as compared to E2A-/- (expression data from ref. 27; ***P<0.0001 by Student t-test, center bars = median, box = 25-75% confidence interval, whiskers = 10-90% confidence interval). (h) IC50 for five Down syndrome-ALLs treated in vitro with GSK-J4 (error bars represent 95% confidence intervals).
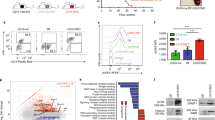
Supplementary Figure 7 HMGN1 overexpression alone results in multiple B cell phenotypes observed with triplication of the entire 21q22 orthologous region.
(a) Relative quantitation of H3K27me3 and HMGN1 in BaF3 lymphoblasts transduced with empty vector of mouse HMGN1. (b) Heatmap showing RNA expression of the 31 triplicated genes in passages 1, 3, and 6 in triplicate Ts1Rhr cultures (blue-red = low to high log2 FPKM values, genes listed in genomic order). (c) Schematic of the primary B cell shRNA experiment. Passage 1 B cells from Ts1Rhr or wild-type bone marrow were pooled after infection with individual lentiviral shRNAs targeting either a triplicated gene (5 shRNA/gene) or a control (n=30). DNA was collected post-infection (baseline) and after each passage (indicated by arrows), and the relative representation of each shRNA was quantitated by next generation sequencing. Data represent the average of independent biological replicates from wild-type (n=3) and Ts1Rhr (n=4) animals. (d) Normalized quantitation of negative (non-targeting) and positive (known to be toxic) control shRNAs in passage 6 Ts1Rhr colonies relative to input (left) demonstrates preferential loss of positive control shRNAs. Neither positive nor negative control shRNAs were preferentially lost from Ts1Rhr passage 3 cells compared to wild-type (right, Tukey box and whiskers plots, horizontal bar is the median and plus is the mean; *P<0.05; NS, not significant). (e) Western blotting in BaF3 lymphoblasts confirming knockdown of HMGN1. Antibodies are: A (Abcam), B (Aviva), mHMGN1 (affinity purified murine HMGN1 antibody). (f) Western blotting of HMGN1 in B cell colonies from wild-type and HMGN_OE mice using the Abcam HMGN1 antibody. “Endo” represents endogenous mouse HMGN1 and “Tg” represents transgenic human HMGN1. (g) Hardy B cell subfractions as percentages of bone marrow cells from wild-type (black) and HMGN1_OE (orange) littermates (n=4 per group, *P<0.05).
Supplementary Figure 8 HMGN1 overexpression cooperates with BCR-ABL to promote B-ALL in vivo.
Results from three independent experiments showing leukemia-free survival of recipients of wild-type (WT) or HMGN1 overexpressing (HMGN1_OE) bone marrow transduced with BCR-ABL (curves compared by log-rank test).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 2 (PDF 3209 kb)
Supplementary Table 1
Differentially expressed genes, gene set enrichment analysis, and shRNA assay results. (XLSX 82 kb)
Rights and permissions
About this article
Cite this article
Lane, A., Chapuy, B., Lin, C. et al. Triplication of a 21q22 region contributes to B cell transformation through HMGN1 overexpression and loss of histone H3 Lys27 trimethylation. Nat Genet 46, 618–623 (2014). https://doi.org/10.1038/ng.2949
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2949
This article is cited by
-
Effects of aneuploidy on cell behaviour and function
Nature Reviews Molecular Cell Biology (2022)
-
H3K27ac nucleosomes facilitate HMGN localization at regulatory sites to modulate chromatin binding of transcription factors
Communications Biology (2022)
-
HMGN1 plays a significant role in CRLF2 driven Down Syndrome leukemia and provides a potential therapeutic target in this high-risk cohort
Oncogene (2022)
-
Loss of grand histone H3 lysine 27 trimethylation domains mediated transcriptional activation in esophageal squamous cell carcinoma
npj Genomic Medicine (2021)
-
Network analysis of Down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19
Scientific Reports (2021)