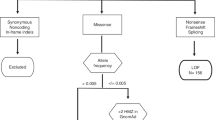
Abstract
Deleterious germline variants in CDKN2A account for around 40% of familial melanoma cases1, and rare variants in CDK4, BRCA2, BAP1 and the promoter of TERT have also been linked to the disease2,3,4,5. Here we set out to identify new high-penetrance susceptibility genes by sequencing 184 melanoma cases from 105 pedigrees recruited in the UK, The Netherlands and Australia that were negative for variants in known predisposition genes. We identified families where melanoma cosegregates with loss-of-function variants in the protection of telomeres 1 gene (POT1), with a proportion of family members presenting with an early age of onset and multiple primary tumors. We show that these variants either affect POT1 mRNA splicing or alter key residues in the highly conserved oligonucleotide/oligosaccharide-binding (OB) domains of POT1, disrupting protein-telomere binding and leading to increased telomere length. These findings suggest that POT1 variants predispose to melanoma formation via a direct effect on telomeres.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Goldstein, A.M. et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J. Med. Genet. 44, 99–106 (2007).
Horn, S. et al. TERT promoter mutations in familial and sporadic melanoma. Science 339, 959–961 (2013).
Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 91, 1310–1316 (1999).
Wiesner, T. et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat. Genet. 43, 1018–1021 (2011).
Zuo, L. et al. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat. Genet. 12, 97–99 (1996).
Law, M.H., Macgregor, S. & Hayward, N.K. Melanoma genetics: recent findings take us beyond well-traveled pathways. J. Invest. Dermatol. 132, 1763–1774 (2012).
Loayza, D. & De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423, 1013–1018 (2003).
Baumann, P. & Cech, T.R. Pot1, the putative telomere end–binding protein in fission yeast and humans. Science 292, 1171–1175 (2001).
Yeo, G. & Burge, C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 11, 377–394 (2004).
Ramsay, A.J. et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat. Genet. 45, 526–530 (2013).
Speedy, H.E. et al. A genome-wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemia. Nat. Genet. 46, 56–60 (2014).
Nandakumar, J., Podell, E.R. & Cech, T.R. How telomeric protein POT1 avoids RNA to achieve specificity for single-stranded DNA. Proc. Natl. Acad. Sci. USA 107, 651–656 (2010).
Lei, M., Podell, E.R. & Cech, T.R. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat. Struct. Mol. Biol. 11, 1223–1229 (2004).
Fu, W. et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 493, 216–220 (2013).
Ding, Z. et al. Estimating telomere length from whole genome sequence data. Nucleic Acids Res. 10.1093/nar/gku181 (7 March 2014).
Burke, L.S. et al. Telomere length and the risk of cutaneous malignant melanoma in melanoma-prone families with and without CDKN2A mutations. PLoS ONE 8, e71121 (2013).
Kendellen, M.F., Barrientos, K.S. & Counter, C.M. POT1 association with TRF2 regulates telomere length. Mol. Cell. Biol. 29, 5611–5619 (2009).
Forbes, S.A. et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 39, D945–D950 (2011).
Gonzalez-Perez, A. et al. IntOGen-mutations identifies cancer drivers across tumor types. Nat. Methods 10, 1081–1082 (2013).
Shi, J. et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat. Genet. 10.1038/ng.2941 (30 March 2014).
Jacobs, J.J. Loss of telomere protection: consequences and opportunities. Front. Oncol. 3, 88 (2013).
Silverman, B.W. Density Estimation (Chapman and Hall, London, 1986).
Ng, P.C. & Henikoff, S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Lopes, M.C. et al. A combined functional annotation score for non-synonymous variants. Hum. Hered. 73, 47–51 (2012).
Aitken, J.F., Green, A.C., MacLennan, R., Youl, P. & Martin, N.G. The Queensland Familial Melanoma Project: study design and characteristics of participants. Melanoma Res. 6, 155–165 (1996).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
McLaren, W. et al. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 26, 2069–2070 (2010).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Waterhouse, A.M., Procter, J.B., Martin, D.M., Clamp, M. & Barton, G.J. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Felsenstein, J. PHYLIP—Phylogeny Inference Package (Version 3.2). Cladistics 5, 164–166 (1989).
Baumann, P., Podell, E. & Cech, T.R. Human Pot1 (protection of telomeres) protein: cytolocalization, gene structure, and alternative splicing. Mol. Cell. Biol. 22, 8079–8087 (2002).
McGrath, M., Wong, J.Y., Michaud, D., Hunter, D.J. & De Vivo, I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol. Biomarkers Prev. 16, 815–819 (2007).
Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, e47 (2002).
Pooley, K.A. et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 70, 3170–3176 (2010).
Bojesen, S.E. et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat. Genet. 45, 371–384 (2013).
Gonzalez-Perez, A. & Lopez-Bigas, N. Functional impact bias reveals cancer drivers. Nucleic Acids Res. 40, e169 (2012).
Acknowledgements
We thank the UK10K Consortium (funded by the Wellcome Trust; WT091310) for access to control data. D.J.A., C.D.R.-E., Z.D., J.Z.L., J.C.T., M.P. and T.M.K. were supported by Cancer Research UK and the Wellcome Trust (WT098051). C.D.R.-E. was also supported by the Consejo Nacional de Ciencia y Tecnología of Mexico. K.A.P. and A.M.D. were supported by Cancer Research UK (grants C1287/A9540 and C8197/A10123) and by the Isaac Newton Trust. N.K.H. was supported by a fellowship from the National Health and Medical Research Council of Australia (NHMRC). L.G.A. was supported by an Australia and New Zealand Banking Group Limited Trustees PhD scholarship. A.L.P. is supported by Cure Cancer Australia. The work was funded in part by the NHMRC and Cancer Council Queensland. The work of N.A.G. was in part supported by the Dutch Cancer Society (UL 2012-5489). M.H., J.A.N.-B. and D.T.B. were supported by Cancer Research UK (programme awards C588/A4994 and C588/A10589 and the Genomics Initiative). C.L.-O., A.J.R. and V.Q. are funded by the Spanish Ministry of Economy and Competitiveness through the Instituto de Salud Carlos III (ISCIII), the Red Temática de Investigación del Cáncer (RTICC) del ISCIII and the Consolider-Ingenio RNAREG Consortium. C.L.-O. is an investigator with the Botín Foundation.
Author information
Authors and Affiliations
Contributions
C.D.R.-E., M.H., J.A.N.-B., D.T.B., N.K.H. and D.J.A. designed the study and wrote the manuscript. C.D.R.-E., M.H., L.G.A., J.C.T., M.M., J.C., M.P., A.J.R., Z.D., V.Q., A.L.P., J.M.P., J.S., M.S.S., N.G.M., M.G.G., A.M.D., K.A.P., P.J., J.Z.L., K.M.B., C.L.-O. and T.M.K. performed experiments or analysis. N.A.G., G.W.M., H.S. and N.G.M. provided vital biological resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Capillary sequencing of POT1 variants detected by next-generation sequencing.
One sample per variant is shown; variants are indicated with an arrow. (a) Sequencing showing the presence of the variant in an additional member of pedigree UF20 who was not exome sequenced (individual III-2; Fig. 1a). (b) Sequencing of an affected individual of pedigree AF1; note that, in this case, the complementary strand is shown. (c) Sequencing of the POT1 product in a control with wild-type POT1 (top) and a carrier of the splice-acceptor variant (pedigree AF1, individual IV-1; Fig. 1a) (bottom). The boundary between exons 17 and 18 is marked with a dotted red line. The wild-type sequence, in nucleotides and in amino acids, is indicated in black, and the sequence the variant results in is indicated in red. The mutant sequence leads to the introduction of a premature stop codon 11 amino acids downstream of the exon 17–exon 18 boundary. (d) Sequencing of an affected member of pedigree UF31. (e) Sequencing of the carrier member of pedigree UF23.
Supplementary Figure 2 MaxEntScan scores for the splice-acceptor variant detected in a familial melanoma pedigree (AF1).
The location of the scores for the wild-type (black) and mutated (red) sequences are shown against score distributions for real splice donors, real splice acceptors and random genomic sequences.
Supplementary Figure 3 Pedigrees with additional POT1 variants.
(a) Pedigree of family UF31 carrying the p.Gln94Glu change. (b) Pedigree of family UF23 carrying the p.Arg273Leu variant. Types of cancer are indicated under each symbol, and ages of onset are indicated in parentheses. Genotypes for all samples available for testing are shown in blue. All cancers were confirmed by histological analysis with the exception of one case (indicated by an asterisk). CMM, cutaneous malignant melanoma. Circles represent females; squares represent males; diamonds represent individuals of undisclosed sex. Half-filled symbols represent other cancers. The individuals that were sequenced have a red outline.
Supplementary Figure 4 Principal-component analysis showing that the melanoma cases and UK10K controls are ancestry matched.
Plot showing the first and second principal components (PC1 and PC2, respectively). Ancestry was estimated using the 1000 Genomes Project individuals1 and then projected onto the melanoma (gray) and UK10K control (orange) cohorts. Note that controls lying greater than 2 s.d. from the mean PC1 or PC2 scores, calculated using only European individuals in the 1000 Genomes Project data set, are not shown in this plot and were not considered in subsequent analyses. We did not depict three individuals from the QFMP cohort for whom we could not determine the zygosity of the called genotypes.
Supplementary Figure 5 35S gel showing the in vitro translation products of wild-type POT1 and OB domain mutants.
This gel confirms that each in vitro translation reaction successfully produced protein for the electrophoretic mobility shift assay shown in Figure 2b. The p.Tyr89Cys, p.Gln94Glu and p.Arg273Leu POT1 variants were identified by exome sequencing familial melanoma cases. The p.Tyr223Cys variant was somatically acquired in CLL and has previously been shown to be unable to bind to telomeric DNA2. The DNA-protein complexes shown in Figure 2b were visualized by 32P labeling of (TTAGGG)3 single-stranded DNA (Online Methods).
Supplementary Figure 6 Linear model used to adjust bioinformatically calculated telomere lengths for age at blood draw and sex.
Residuals were used as the adjusted relative telomere lengths. Dark circles represent male samples, and light circles represent females. The sex variable is coded as 0 = female, 1 = male. Note that only two dimensions (relative telomere length and age) are shown.
Supplementary Figure 7 Linear model used to adjust PCR mean ΔCt values for age at blood draw and sex.
Residuals were used as the adjusted mean ΔCt values. Dark circles represent male samples, and light circles represent females. The sex variable is coded as 0 = female, 1 = male. Note that only two dimensions (mean ΔCt value and age) are shown.
Supplementary Figure 8 PCR-based estimate of telomere length showing the carrier of a POT1 intronic splice-donor variant.
The carrier of the intronic splice-donor variant is shown in the fourth row in green. The rest of the figure is identical to Figure 2d.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1, 2 and 4–8 and Supplementary Note (PDF 2264 kb)
Supplementary Table 3
Genes with cosegregating variants from the 28 pedigrees for which we had sequence data for 3 or more members and their GO terms. (XLS 159 kb)
Rights and permissions
About this article
Cite this article
Robles-Espinoza, C., Harland, M., Ramsay, A. et al. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet 46, 478–481 (2014). https://doi.org/10.1038/ng.2947
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2947