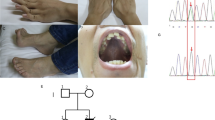
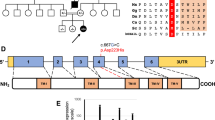
Abstract
Mitochondrial Ca2+ uptake has key roles in cell life and death. Physiological Ca2+ signaling regulates aerobic metabolism, whereas pathological Ca2+ overload triggers cell death. Mitochondrial Ca2+ uptake is mediated by the Ca2+ uniporter complex in the inner mitochondrial membrane1,2, which comprises MCU, a Ca2+-selective ion channel, and its regulator, MICU1. Here we report mutations of MICU1 in individuals with a disease phenotype characterized by proximal myopathy, learning difficulties and a progressive extrapyramidal movement disorder. In fibroblasts from subjects with MICU1 mutations, agonist-induced mitochondrial Ca2+ uptake at low cytosolic Ca2+ concentrations was increased, and cytosolic Ca2+ signals were reduced. Although resting mitochondrial membrane potential was unchanged in MICU1-deficient cells, the mitochondrial network was severely fragmented. Whereas the pathophysiology of muscular dystrophy3 and the core myopathies4 involves abnormal mitochondrial Ca2+ handling, the phenotype associated with MICU1 deficiency is caused by a primary defect in mitochondrial Ca2+ signaling, demonstrating the crucial role of mitochondrial Ca2+ uptake in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011).
Perocchi, F. et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467, 291–296 (2010).
Millay, D.P. et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 14, 442–447 (2008).
Boncompagni, S. et al. Characterization and temporal development of cores in a mouse model of malignant hyperthermia. Proc. Natl. Acad. Sci. USA 106, 21996–22001 (2009).
Duchen, M.R. & Szabadkai, G. Roles of mitochondria in human disease. Essays Biochem. 47, 115–137 (2010).
McCormack, J.G., Halestrap, A.P. & Denton, R.M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70, 391–425 (1990).
Szabadkai, G. & Duchen, M.R. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 23, 84–94 (2008).
Rizzuto, R. & Pozzan, T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369–408 (2006).
Giacomello, M., Drago, I., Pizzo, P. & Pozzan, T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 14, 1267–1274 (2007).
Csordás, G. et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 17, 976–987 (2013).
Plovanich, M. et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE 8, e55785 (2013).
Mallilankaraman, K. et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 151, 630–644 (2012).
Baughman, J.M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011).
Carr, I.M., Sheridan, E., Hayward, B.E., Markham, A.F. & Bonthron, D.T. IBDfinder and SNPsetter: tools for pedigree-independent identification of autozygous regions in individuals with recessive inherited disease. Hum. Mutat. 30, 960–967 (2009).
Stevens, E. et al. Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of α-dystroglycan. Am. J. Hum. Genet. 92, 354–365 (2013).
Zampese, E. et al. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. USA 108, 2777–2782 (2011).
Chami, M. et al. Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol. Cell 32, 641–651 (2008).
Bianchi, K., Rimessi, A., Prandini, A., Szabadkai, G. & Rizzuto, R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim. Biophys. Acta 1742, 119–131 (2004).
Gerencsér, A.A. & Adam-Vizi, V. Selective, high-resolution fluorescence imaging of mitochondrial Ca2+ concentration. Cell Calcium 30, 311–321 (2001).
Spät, A. & Pitter, J.G. The effect of cytoplasmic Ca2+ signal on the redox state of mitochondrial pyridine nucleotides. Mol. Cell Endocrinol. 215, 115–118 (2004).
Irwin, W.A. et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat. Genet. 35, 367–371 (2003).
Dubowitz, V., Sewry, C.A. & Oldfors, A. Muscle Biopsy: A Practical Approach 4th edn. (Saunders, Philadelphia, 2013).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386 (2000).
Dawe, H.R. et al. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum. Mol. Genet. 16, 173–186 (2007).
Bolte, S. & Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006).
Traba, J., Del Arco, A., Duchen, M.R., Szabadkai, G. & Satrustegui, J. SCaMC-1 promotes cancer cell survival by desensitizing mitochondrial permeability transition via ATP/ADP-mediated matrix Ca2+ buffering. Cell Death Differ. 19, 650–660 (2012).
Chen, T.W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Maravall, M., Mainen, Z.F., Sabatini, B.L. & Svoboda, K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys. J. 78, 2655–2667 (2000).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A.G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160 (2009).
Acknowledgements
This work was supported by a Sir Jules Thorn Award for Biomedical Research (JTA/09 to C.A.J., E.S. and D.T.B.). We acknowledge funding from the Medical Research Council (project grant MR/K011154/1) awarded to C.A.J., E.S. and C.V.L. We are grateful to the UK10K Consortium for making this study possible. This study makes use of data generated by the UK10K Consortium. A full list of the investigators who contributed to the generation of the data is available at http://www.uk10k.org/publications.html. Funding for UK10K was provided by the Wellcome Trust under award WT091310. Muscular Dystrophy Association grant 68762 to F.M. is gratefully acknowledged. National Specialised Commissioned Team (NSCT) funding for the Congenital Muscular Dystrophies and Congenital Myopathy service in London is also gratefully acknowledged. T.W. is a Muscular Dystrophy Campaign PhD student. F.M. is supported by the Great Ormond Street Children's Charity and the Great Ormond Street Hospital Biomedical Research Centre. This study was also partly supported by the MRC Neuromuscular Centre biobank. European Union Framework Programme 7 Neuromic grant to G.-J. van Ommen (FP7-Health-2012-Innovation 1 HEALTH-F5-2012-305121) is also gratefully acknowledged. G.S. is supported by Parkinson's UK, the British Heart Foundation, the Wellcome Trust–UCL Therapeutic Innovation Fund, Telethon-Italy (GEP1206) and the Italian Association for Cancer Research (AIRC). R.R. is supported by the Italian Ministries of Health (Ricerca Finalizzata) and of Education, University and Research (PRIN and FIRB), the European Union (European Research Council mitoCalcium, 294777), the US National Institutes of Health (NIH; grant 1P01AG025532-01A1), the Cariparo Foundation and the Cariplo Foundations (Padua), AIRC and Telethon-Italy (GPP1005A and GPP11082B). J.A.S. is the recipient of a PhD studentship from the Muscular Dystrophy Campaign. The genealogy of family UMCU was constructed by F.A.M. Hennekam (University Medical Center Utrecht).
Author information
Authors and Affiliations
Consortia
Contributions
E.S., F.M., M.R.D. and G.S. designed the study and experiments. E.S., F.M., A.-M.C., K.A.P., D.T.B., M.K., E.H.N., W.L.v.d.P., D.L., G.W.E.S. and H.R. identified, consented and recruited the study subjects and provided clinical information. D.A.P., C.V.L., C.A.J., J.E.M., M.K., I.G., J.T.d.D., Y.S. and M.H. generated and analyzed clonal sequencing data. C.V.L., D.A.P., G.W., N.Y.R., K.S., C.A.J., S.N., Z.A.A., T.W. and S.T. performed genetic analysis, confirmation studies and haplotyping. J.A.S., G.S., D.D.S., A.R., R.R. and M.R.D. performed calcium handling, cellular imaging, oxygen consumption and protein expression studies. C.A.S., R.P., I.M. and A.R.F. undertook muscle immunohistochemistry and analysis of patient muscle biopsies. E.S., C.V.L., D.T.B., F.M., G.S. and M.R.D. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A full list of members and affiliations appear in the Supplementary Note.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Tables 1–4 and Supplementary Figures 1–15 (PDF 7587 kb)
Rights and permissions
About this article
Cite this article
Logan, C., Szabadkai, G., Sharpe, J. et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet 46, 188–193 (2014). https://doi.org/10.1038/ng.2851
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2851