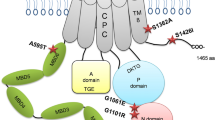
Abstract
Type II P-type ATPases (PAIIs) constitute a family of conserved proteins that actively generate ionic gradients across membranes. Mutations in genes encoding PAIIs can cause heritable dominant diseases, with suggested etiology of haploinsufficiency. Using a Drosophila melanogaster genetic screen, we identified a dominant mutation altering the PAII member sarcoendoplasmic reticulum Ca2+ ATPase (SERCA). This mutation conferred temperature-sensitive uncoordination in a gain-of-function manner. We established that this gain-of-function phenotype is linked to dominant disease-causing mutations affecting various human PAIIs. We further found that heterologous expression of mutant PAIIs elicited ion leakage that was exacerbated at elevated temperatures. Therefore, these dominant mutations result in ionic leakage and render PAIIs susceptible to deleterious effects from elevated temperatures. Accordingly, it was recently reported that missense mutations affecting the Na+/K+ ATPase can elicit ionic leakage. We propose that ionic leakage is a pervasive gain-of-function mechanism that can underlie a variety of dominant PAII-related diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kühlbrandt, W. Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 5, 282–295 (2004).
Palmgren, M.G. & Nissen, P. P-type ATPases. Annu. Rev. Biophys. 40, 243–266 (2011).
Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000).
Toyoshima, C., Kanai, R. & Cornelius, F. First crystal structures of Na+,K+-ATPase: new light on the oldest ion pump. Structure 19, 1732–1738 (2011).
Bublitz, M., Poulsen, H., Morth, J.P. & Nissen, P. In and out of the cation pumps: P-type ATPase structure revisited. Curr. Opin. Struct. Biol. 20, 431–439 (2010).
Szigeti, R. & Kellermayer, R. Autosomal-dominant calcium ATPase disorders. J. Invest. Dermatol. 126, 2370–2376 (2006).
Brini, M. & Carafoli, E. Calcium pumps in health and disease. Physiol. Rev. 89, 1341–1378 (2009).
de Vries, B., Frants, R.R., Ferrari, M.D. & van den Maagdenberg, A.M. Molecular genetics of migraine. Hum. Genet. 126, 115–132 (2009).
de Carvalho Aguiar, P. et al. Mutations in the Na+/K+-ATPase α3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43, 169–175 (2004).
Heinzen, E.L. et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat. Genet. 44, 1030–1034 (2012).
Brashear, A., Sweadner, K. & Ozelius, L. in GeneReviews (eds. Pagon, R.A., Bird, T.D., Dolan, C.R., Stephens, K. & Adam, M.P.) (University of Washington, Seattle, 1993).
De Fusco, M. et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 33, 192–196 (2003).
Lingrel, J.B., Williams, M.T., Vorhees, C.V. & Moseley, A.E. Na,K-ATPase and the role of α isoforms in behavior. J. Bioenerg. Biomembr. 39, 385–389 (2007).
Moseley, A.E. et al. Deficiency in Na,K-ATPase α isoform genes alters spatial learning, motor activity, and anxiety in mice. J. Neurosci. 27, 616–626 (2007).
Okunade, G.W. et al. Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J. Biol. Chem. 282, 26517–26527 (2007).
Shull, G.E. et al. Physiological functions of plasma membrane and intracellular Ca2+ pumps revealed by analysis of null mutants. Ann. NY Acad. Sci. 986, 453–460 (2003).
Beuschlein, F. et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 45, 440–444 (2013).
Azizan, E.A. et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 45, 1055–1060 (2013).
Cook, B., Hardy, R.W., McConnaughey, W.B. & Zuker, C.S. Preserving cell shape under environmental stress. Nature 452, 361–364 (2008).
Koundakjian, E.J., Cowan, D.M., Hardy, R.W. & Becker, A.H. The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics 167, 203–206 (2004).
Sanyal, S. et al. Analysis of conditional paralytic mutants in Drosophila sarco-endoplasmic reticulum calcium ATPase reveals novel mechanisms for regulating membrane excitability. Genetics 169, 737–750 (2005).
Riant, F. et al. ATP1A2 mutations in 11 families with familial hemiplegic migraine. Hum. Mutat. 26, 281 (2005).
Podestà, B., Briatore, E., Boghi, A., Marenco, D. & Calzolari, S. Transient nonverbal learning disorder in a child suffering from Familial Hemiplegic Migraine. Cephalalgia 31, 1497–1502 (2011).
Jen, J.C. et al. Prolonged hemiplegic episodes in children due to mutations in ATP1A2. J. Neurol. Neurosurg. Psychiatry 78, 523–526 (2007).
Godic, A., Korosec, B., Miljkovic, J., Kansky, A. & Glavac, D. Four novel ATP2A2 mutations in Slovenian patients with Darier disease. J. Am. Acad. Dermatol. 62, 819–823 (2010).
Ikeda, S. et al. Mutations in ATP2A2 in patients with Darier's disease. J. Invest. Dermatol. 121, 475–477 (2003).
Green, E.K. et al. Novel ATP2A2 mutations in a large sample of individuals with Darier disease. J. Dermatol. 40, 259–266 (2013).
Jurkat-Rott, K. et al. Variability of familial hemiplegic migraine with novel A1A2 Na+/K+-ATPase variants. Neurology 62, 1857–1861 (2004).
Thomsen, L.L. et al. The genetic spectrum of a population-based sample of familial hemiplegic migraine. Brain 130, 346–356 (2007).
Brashear, A. et al. ATP1A3 mutations in infants: a new rapid-onset dystonia-Parkinsonism phenotype characterized by motor delay and ataxia. Dev. Med. Child Neurol. 54, 1065–1067 (2012).
Chao, S.C., Yang, M.H. & Lee, J.Y. Mutation analysis of the ATP2A2 gene in Taiwanese patients with Darier's disease. Br. J. Dermatol. 146, 958–963 (2002).
Fu, X., Liu, H., Yan, X., Yu, Y. & Zhang, F. Mutation analysis of the ATP2A2 gene in Chinese patients with Darier's disease. J. Eur. Acad. Dermatol. Venereol. 25, 370–371 (2011).
Ren, Y.Q. et al. Five mutations of ATP2A2 gene in Chinese patients with Darier's disease and a literature review of 86 cases reported in China. Arch. Dermatol. Res. 298, 58–63 (2006).
Ringpfeil, F. et al. Darier disease—novel mutations in ATP2A2 and genotype-phenotype correlation. Exp. Dermatol. 10, 19–27 (2001).
Sakuntabhai, A., Burge, S., Monk, S. & Hovnanian, A. Spectrum of novel ATP2A2 mutations in patients with Darier's disease. Hum. Mol. Genet. 8, 1611–1619 (1999).
Onozuka, T., Sawamura, D., Yokota, K. & Shimizu, H. Mutational analysis of the ATP2A2 gene in two Darier disease families with intrafamilial variability. Br. J. Dermatol. 150, 652–657 (2004).
Wang, P.G. et al. Genetic heterogeneity in acrokeratosis verruciformis of Hopf. Clin. Exp. Dermatol. 31, 558–563 (2006).
Gadsby, D.C. Ion channels versus ion pumps: the principal difference, in principle. Nat. Rev. Mol. Cell Biol. 10, 344–352 (2009).
Ruiz-Perez, V.L. et al. ATP2A2 mutations in Darier's disease: variant cutaneous phenotypes are associated with missense mutations, but neuropsychiatric features are independent of mutation class. Hum. Mol. Genet. 8, 1621–1630 (1999).
Sakuntabhai, A. et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat. Genet. 21, 271–277 (1999).
Ahn, W., Lee, M.G., Kim, K.H. & Muallem, S. Multiple effects of SERCA2b mutations associated with Darier's disease. J. Biol. Chem. 278, 20795–20801 (2003).
Duman, J.G., Chen, L. & Hille, B. Calcium transport mechanisms of PC12 cells. J. Gen. Physiol. 131, 307–323 (2008).
Dode, L. et al. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J. Biol. Chem. 278, 47877–47889 (2003).
Miyauchi, Y. et al. Comprehensive analysis of expression and function of 51 sarco(endo)plasmic reticulum Ca2+-ATPase mutants associated with Darier disease. J. Biol. Chem. 281, 22882–22895 (2006).
Tavraz, N.N. et al. Diverse functional consequences of mutations in the Na+/K+-ATPase α2-subunit causing familial hemiplegic migraine type 2. J. Biol. Chem. 283, 31097–31106 (2008).
Isken, O. & Maquat, L.E. The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nat. Rev. Genet. 9, 699–712 (2008).
Leo, L. et al. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 7, e1002129 (2011).
Clapcote, S.J. et al. Mutation I810N in the α3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc. Natl. Acad. Sci. USA 106, 14085–14090 (2009).
DeAndrade, M.P., Yokoi, F., van Groen, T., Lingrel, J.B. & Li, Y. Characterization of Atp1a3 mutant mice as a model of rapid-onset dystonia with parkinsonism. Behav. Brain Res. 216, 659–665 (2011).
Kirshenbaum, G.S. et al. Alternating hemiplegia of childhood–related neural and behavioural phenotypes in Na+,K+-ATPase α3 missense mutant mice. PLoS ONE 8, e60141 (2013).
Ishii, A. et al. Identification of ATP1A3 mutations by exome sequencing as the cause of alternating hemiplegia of childhood in Japanese patients. PLoS ONE 8, e56120 (2013).
Rosewich, H. et al. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol. 11, 764–773 (2012).
Artigas, P. & Gadsby, D.C. Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels. Proc. Natl. Acad. Sci. USA 100, 501–505 (2003).
Yaragatupalli, S., Olivera, J.F., Gatto, C. & Artigas, P. Altered Na+ transport after an intracellular α-subunit deletion reveals strict external sequential release of Na+ from the Na/K pump. Proc. Natl. Acad. Sci. USA 106, 15507–15512 (2009).
Meier, S., Tavraz, N.N., Durr, K.L. & Friedrich, T. Hyperpolarization-activated inward leakage currents caused by deletion or mutation of carboxy-terminal tyrosines of the Na+/K+-ATPase α subunit. J. Gen. Physiol. 135, 115–134 (2010).
Nussinov, R. & Tsai, C.J. Allostery in disease and in drug discovery. Cell 153, 293–305 (2013).
Cahalan, M.D. STIMulating store-operated Ca2+ entry. Nat. Cell Biol. 11, 669–677 (2009).
Hill, J.K. et al. Splice-site A choice targets plasma-membrane Ca2+-ATPase isoform 2 to hair bundles. J. Neurosci. 26, 6172–6180 (2006).
Acknowledgements
We thank P. Gillespie (Oregon Health and Science University), T. Avidor-Reiss (University of Toledo), A. Kiger (University of California, San Diego), S. Courtneidge (Sanford-Burnham Medical Research Institute) and L. Luo (Stanford University) for reagents used in this work. We thank K. Spencer for technical assistance and A. Chadha for comments on the manuscript. The Uncts2 mutant was identified by screening a collection of F3 Drosophila lines in the laboratory of C. Zuker.
Author information
Authors and Affiliations
Contributions
B.C. performed mutagenesis screening and mapped the revertant lines. B.S.D. cloned and sequenced the dSERCA gene from the Uncts2 mutant. M.K. generated and tested all transgenic lines, sequenced dSERCA from additional temperature-sensitive uncoordinated mutants and performed the calcium-imaging experiments in cultured cells. B.C. led the entire project. M.K. generated tables and figures. M.K. and B.C. wrote the manuscript. All authors participated in the discussion and interpretation of data and results, and all participated in the editing and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2, and Supplementary Figures 1–4 (PDF 1324 kb)
Rights and permissions
About this article
Cite this article
Kaneko, M., Desai, B. & Cook, B. Ionic leakage underlies a gain-of-function effect of dominant disease mutations affecting diverse P-type ATPases. Nat Genet 46, 144–151 (2014). https://doi.org/10.1038/ng.2850
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2850
This article is cited by
-
ATP1A3-related phenotypes in Chinese children: AHC, CAPOS, and RECA
European Journal of Pediatrics (2022)
-
Fever-related ataxia: a case report of CAPOS syndrome
Cerebellum & Ataxias (2019)
-
Characterization of Hailey-Hailey Disease-mutants in presence and absence of wild type SPCA1 using Saccharomyces cerevisiae as model organism
Scientific Reports (2019)
-
SERCA is critical to control the Bowditch effect in the heart
Scientific Reports (2018)
-
Pathways to neurodegeneration: lessons learnt from unbiased genetic screens in Drosophila
Journal of Genetics (2018)