Abstract
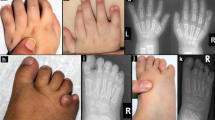
Lenz-Majewski syndrome (LMS) is a syndrome of intellectual disability and multiple congenital anomalies that features generalized craniotubular hyperostosis. By using whole-exome sequencing and selecting variants consistent with the predicted dominant de novo etiology of LMS, we identified causative heterozygous missense mutations in PTDSS1, which encodes phosphatidylserine synthase 1 (PSS1). PSS1 is one of two enzymes involved in the production of phosphatidylserine. Phosphatidylserine synthesis was increased in intact fibroblasts from affected individuals, and end-product inhibition of PSS1 by phosphatidylserine was markedly reduced. Therefore, these mutations cause a gain-of-function effect associated with regulatory dysfunction of PSS1. We have identified LMS as the first human disease, to our knowledge, caused by disrupted phosphatidylserine metabolism. Our results point to an unexplored link between phosphatidylserine synthesis and bone metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Braham, R.L. Multiple congenital abnormalities with diaphyseal dysplasia (Ca murati-Engelmann's syndrome). Report of a case. Oral Surg. Oral Med. Oral Pathol. 27, 20–26 (1969).
Macpherson, R.I. Craniodiaphyseal dysplasia, a disease or group of diseases? J. Can. Assoc. Radiol. 25, 22–33 (1974).
Kaye, C.I., Fisher, D.E. & Esterly, N.B. Cutis laxa, skeletal anomalies, and ambiguous genitalia. Am. J. Dis. Child. 127, 115–117 (1974).
Lenz, W.D. & Majewski, F. A generalized disorders of the connective tissues with progeria, choanal atresia, symphalangism, hypoplasia of dentine and craniodiaphyseal hypostosis. Birth Defects Orig. Artic. Ser. 10, 133–136 (1974).
Majewski, F. Lenz-Majewski hyperostotic dwarfism: reexamination of the original patient. Am. J. Med. Genet. 93, 335–338 (2000).
Robinow, M., Johanson, A.J. & Smith, T.H. The Lenz-Majewski hyperostotic dwarfism. A syndrome of multiple congenital anomalies, mental retardation, and progressive skeletal sclerosis. J. Pediatr. 91, 417–421 (1977).
Gorlin, R.J. & Whitley, C.B. Lenz-Majewski syndrome. Radiology 149, 129–131 (1983).
Chrzanowska, K.H., Fryns, J.P., Krajewska-Walasek, M., Van den Berghe, H. & Wisniewski, L. Skeletal dysplasia syndrome with progeroid appearance, characteristic facial and limb anomalies, multiple synostoses, and distinct skeletal changes: a variant example of the Lenz-Majewski syndrome. Am. J. Med. Genet. 32, 470–474 (1989).
Saraiva, J.M. Dysgenesis of corpus callosum in Lenz-Majewski hyperostotic dwarfism. Am. J. Med. Genet. 91, 198–200 (2000).
Wattanasirichaigoon, D., Visudtibhan, A., Jaovisidha, S., Laothamatas, J. & Chunharas, A. Expanding the phenotypic spectrum of Lenz-Majewski syndrome: facial palsy, cleft palate and hydrocephalus. Clin. Dysmorphol. 13, 137–142 (2004).
Sherry, S.T. et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 29, 308–311 (2001).
1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Vance, J.E. & Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 1831, 543–554 (2013).
Tomohiro, S., Kawaguti, A., Kawabe, Y., Kitada, S. & Kuge, O. Purification and characterization of human phosphatidylserine synthases 1 and 2. Biochem. J. 418, 421–429 (2009).
Leventis, P.A. & Grinstein, S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 39, 407–427 (2010).
Mozzi, R., Buratta, S. & Goracci, G. Metabolism and functions of phosphatidylserine in mammalian brain. Neurochem. Res. 28, 195–214 (2003).
Schick, P.K., Kurica, K.B. & Chacko, G.K. Location of phosphatidylethanolamine and phosphatidylserine in the human platelet plasma membrane. J. Clin. Invest. 57, 1221–1226 (1976).
Saito, K., Nishijima, M. & Kuge, O. Genetic evidence that phosphatidylserine synthase II catalyses the conversion of phosphatidylethanolamine to phosphatidylserine in Chinese Hamster Ovary cells. Biochemistry 273, 17199–17205 (1998).
Stone, S.J. & Vance, J.E. Cloning and expression of murine liver phosphatidylserine synthase (PSS)-2: differential regulation of phospholipid metabolism by PSS1 and PSS2. Biochem. J. 342, 57–64 (1999).
Ohsawa, T., Nishijima, M. & Kuge, O. Functional analysis of Chinese hamster phosphatidylserine synthase 1 through systematic alanine mutagenesis. Biochem. J. 381, 853–859 (2004).
Arikketh, D., Nelson, R. & Vance, J.E. Defining the importance of phosphatidylserine synthase-1 (PSS1): unexpected viability of PSS1-deficient mice. J. Biol. Chem. 283, 12888–12897 (2008).
Bergo, M.O. et al. Defining the importance of phosphatidylserine synthase 2 in mice. J. Biol. Chem. 277, 47701–47708 (2002).
Allanson, J. et al. Nablus mask-like facial syndrome: deletion of chromosome 8q22.1 is necessary but not sufficient to cause the phenotype. Am. J. Med. Genet. A. 158A, 2091–2099 (2012).
Nishijimas, M., Kuge, O. & Akamatsu, Y. Phosphatidylserine biosynthesis in cultured Chinese hamster ovary cells. I. Inhibition of de novo phosphatidylserine biosynthesis by exogenous phosphatidylserine and its efficient incorporation. J. Biol. Chem. 261, 5784–5789 (1986).
Kuge, O., Saito, K. & Nishijima, M. Control of phosphatidylserine synthase II activity in Chinese hamster ovary cells. J. Biol. Chem. 274, 23844–23849 (1999).
Kuge, O., Hasegawa, K., Saito, K. & Nishijima, M. Control of phosphatidylserine biosynthesis through phosphatidylserine-mediated inhibition of phosphatidylserine synthase I in Chinese hamster ovary cells. Proc. Natl. Acad. Sci. USA 95, 4199–4203 (1998).
Morita, S.-Y. et al. Enzymatic measurement of phosphatidylserine in cultured cells. J. Lipid Res. 53, 325–330 (2012).
Stone, S.J., Cui, Z. & Vance, J.E. Cloning and expression of mouse liver phosphatidylserine synthase-1 cDNA. J. Biol. Chem. 273, 7293–7302 (1998).
Gerety, S.S. & Wilkinson, D.G. Morpholino artifacts provide pitfalls and reveal a novel role for pro-apoptotic genes in hindbrain boundary development. Dev. Biol. 350, 279–289 (2011).
Sturbois-Balcerzak, B., Stone, S.J., Sreenivas, A. & Vance, J.E. Structure and expression of the murine phosphatidylserine synthase-1 gene. J. Biol. Chem. 276, 8205–8212 (2001).
Tasseva, G., Cole, L. & Vance, J.E. N-Myc and SP regulate phosphatidylserine synthase-1 expression in brain and glial cells. J. Biol. Chem. 286, 1061–1073 (2011).
Kingsley, M. Effects of phosphatidylserine supplementation on exercising humans. Sports Med. 36, 657–669 (2006).
Serby, M.J., Burns, S.J. & Roane, D.M. Treatment of memory loss with herbal remedies. Curr. Treat. Options Neurol. 13, 520–528 (2011).
Mohamed, M. et al. Metabolic cutis laxa syndromes. J. Inherit. Metab. Dis. 34, 907–916 (2011).
Reversade, B. et al. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat. Genet. 41, 1016–1021 (2009).
Hucthagowder, V. et al. Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Hum. Mol. Genet. 18, 2149–2165 (2009).
Fischer, B. et al. Further characterization of ATP6V0A2-related autosomal recessive cutis laxa. Hum. Genet. 131, 1761–1773 (2012).
Warman, M.L. et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am. J. Med. Genet. A. 155A, 943–968 (2011).
Wu, L.N.Y., Genge, B.R. & Wuthier, R.E. Analysis and molecular modeling of the formation, structure, and activity of the phosphatidylserine-calcium-phosphate complex associated with biomineralization. J. Biol. Chem. 283, 3827–3838 (2008).
Merolli, A. & Santin, M. Role of phosphatidyl-serine in bone repair and its technological exploitation. Molecules 14, 5367–5381 (2009).
Thouverey, C., Strzelecka-Kiliszek, A., Balcerzak, M., Buchet, R. & Pikula, S. Matrix vesicles originate from apical membrane microvilli of mineralizing osteoblast-like Saos-2 cells. J. Cell. Biochem. 106, 127–138 (2009).
Geneviève, D. et al. Thromboxane synthase mutations in an increased bone density disorder (Ghosal syndrome). Nat. Genet. 40, 284–286 (2008).
Chen, I.-P., Wang, L., Jiang, X., Aguila, H.L. & Reichenberger, E.J.A. Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Hum. Mol. Genet. 20, 948–961 (2011).
Wu, Z., Ma, H.M., Kukita, T., Nakanishi, Y. & Nakanishi, H. Phosphatidylserine-containing liposomes inhibit the differentiation of osteoclasts and trabecular bone loss. J. Immunol. 184, 3191–3201 (2010).
Stone, S.J. & Vance, J.E. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J. Biol. Chem. 275, 34534–34540 (2000).
Bligh, E.G. & Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Rouser, G., Siakotos, A.N. & Fleischer, S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids 1, 85–86 (1966).
Lindsay, S. & Copp, A.J. MRC-Wellcome Trust Human Developmental Biology Resource: enabling studies of human developmental gene expression. Trends Genet. 21, 586–590 (2005).
Carney, T.J. et al. A direct role for Sox10 in specification of neural crest–derived sensory neurons. Development 133, 4619–4630 (2006).
Acknowledgements
We are grateful to the individuals with LMS and their families for their participation in this study. We thank P. Clayton and R. Hennekam for their comments on the manuscript, A. Offiah for confirming the radiological diagnosis in subject 1 and W. Emmett for his valuable help in processing raw sequencing data. The authors would like to acknowledge D. Trabzuni and the UK Brain Expression Consortium, the Human Brain Transcriptome project at the Department of Neurobiology at the Yale University School of Medicine NHLBI for the use of data from their public database and the GO Exome Sequencing Project and its ongoing studies, which produced and provided exome variant calls for comparison, including the Lung GO Sequencing Project (HL-102923), the Women's Health Initiative (WHI) Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010). S.B.S. was supported by the Fundação para a Ciência e Tecnologia (SFRH/BD/46778/2008). The Centre for Translational Genomics–GOSgene is supported by the Biomedical Research Centre of the National Institute for Health Research at Great Ormond Street Hospital for Children NHS Foundation Trust and UCL Institute of Child Health. D.J. was supported by a Wellcome Trust ViP Award. D.W. is a recipient of Research Career Development Awards from the Faculty of Medicine at Ramathibodi Hospital. M.S. is supported by Czech research development grant 00064203. P.S. is supported by the Great Ormond Street Hospital Children's Charity (GOSHCC). The Fetal Growth and Development research team of G.E.M. is funded by WellBeing of Women, the Wellcome Trust, GOSHCC and the UK Medical Research Council. The research of J.E.V. is supported by the National Science and Engineering Council of Canada.
Author information
Authors and Affiliations
Contributions
S.B.S. and G.E.M. designed the study. S.B.S., A.B., R.S., A.C., J.S., J.M.S., D.W., K.C., M.S. and L.V.M. characterized individuals with LMS and collected clinical data and samples. S.B.S. designed and performed direct mutagenesis, zebrafish experiments, Sanger sequencing and cell culture and wrote the manuscript. D.J. designed and performed zebrafish experiments and wrote the manuscript. E.C. designed and performed next-generation sequencing data analysis and segregation studies and wrote the manuscript. J.E.V. and G.T. designed and performed phospholipid metabolism studies and wrote the manuscript. M.I. performed RT-qPCR experiments. G.A. performed electron microscopy studies. M.R. contributed data for human adult brain transcriptome analysis. J.D. and P.S. performed Annexin V staining experiments. P.S. and P.L.B. contributed to experimental design and data analysis. All authors discussed the results and implications of the work and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–4 and Supplementary Figures 1–11 (PDF 2655 kb)
Supplementary Data Set
Supplementary Data Set (XLSX 73 kb)
Rights and permissions
About this article
Cite this article
Sousa, S., Jenkins, D., Chanudet, E. et al. Gain-of-function mutations in the phosphatidylserine synthase 1 (PTDSS1) gene cause Lenz-Majewski syndrome. Nat Genet 46, 70–76 (2014). https://doi.org/10.1038/ng.2829
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2829
This article is cited by
-
Major skull manifestations of skeletal dysplasias — pictorial essay
Pediatric Radiology (2020)
-
Distribution, dynamics and functional roles of phosphatidylserine within the cell
Cell Communication and Signaling (2019)
-
Lipids and synaptic functions
Journal of Inherited Metabolic Disease (2018)
-
Human Genetics of Sclerosing Bone Disorders
Current Osteoporosis Reports (2018)
-
RYR2, PTDSS1 and AREG genes are implicated in a Lebanese population-based study of copy number variation in autism
Scientific Reports (2016)