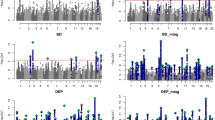
Abstract
Most psychiatric disorders are moderately to highly heritable. The degree to which genetic variation is unique to individual disorders or shared across disorders is unclear. To examine shared genetic etiology, we use genome-wide genotype data from the Psychiatric Genomics Consortium (PGC) for cases and controls in schizophrenia, bipolar disorder, major depressive disorder, autism spectrum disorders (ASD) and attention-deficit/hyperactivity disorder (ADHD). We apply univariate and bivariate methods for the estimation of genetic variation within and covariation between disorders. SNPs explained 17–29% of the variance in liability. The genetic correlation calculated using common SNPs was high between schizophrenia and bipolar disorder (0.68 ± 0.04 s.e.), moderate between schizophrenia and major depressive disorder (0.43 ± 0.06 s.e.), bipolar disorder and major depressive disorder (0.47 ± 0.06 s.e.), and ADHD and major depressive disorder (0.32 ± 0.07 s.e.), low between schizophrenia and ASD (0.16 ± 0.06 s.e.) and non-significant for other pairs of disorders as well as between psychiatric disorders and the negative control of Crohn's disease. This empirical evidence of shared genetic etiology for psychiatric disorders can inform nosology and encourages the investigation of common pathophysiologies for related disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kendler, K.S. & Eaves, L.J. Psychiatric Genetics (Review of Psychiatry) (American Psychiatric Association, Arlington, VA, 2005).
Tsuang, M. & Faraone, S. The Genetics of Mood Disorders (Johns Hopkins University Press, Baltimore, MD, 1990).
Smoller, J.W. & Finn, C.T. Family, twin, and adoption studies of bipolar disorder. Am. J. Med. Genet. C. Semin. Med. Genet. 123C, 48–58 (2003).
Ronald, A., Simonoff, E., Kuntsi, J., Asherson, P. & Plomin, R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J. Child Psychol. Psychiatry 49, 535–542 (2008).
Rommelse, N.N., Franke, B., Geurts, H.M., Hartman, C.A. & Buitelaar, J.K. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur. Child Adolesc. Psychiatry 19, 281–295 (2010).
Lichtenstein, P., Carlstrom, E., Rastam, M., Gillberg, C. & Anckarsater, H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am. J. Psychiatry 167, 1357–1363 (2010).
Rapoport, J., Chavez, A., Greenstein, D., Addington, A. & Gogtay, N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J. Am. Acad. Child Adolesc. Psychiatry 48, 10–18 (2009).
King, B.H. & Lord, C. Is schizophrenia on the autism spectrum? Brain Res. 1380, 34–41 (2011).
Sullivan, P.F. et al. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch. Gen. Psychiatry 69, 1099–1103 (2012).
Crespi, B., Stead, P. & Elliot, M. Comparative genomics of autism and schizophrenia. Proc. Natl. Acad. Sci. USA 107, 1736–1741 (2010).
Mortensen, P.B., Pedersen, M.G. & Pedersen, C.B. Psychiatric family history and schizophrenia risk in Denmark: which mental disorders are relevant? Psychol. Med. 40, 201–210 (2010).
Faraone, S.V., Biederman, J. & Wozniak, J. Examining the comorbidity between attention deficit hyperactivity disorder and bipolar disorder: a meta-analysis of family-genetic studies. Am. J. Psychiatry 169, 1256–1266 (2012).
Cole, J., Ball, H.A., Martin, N.C., Scourfield, J. & McGuffin, P. Genetic overlap between measures of hyperactivity/inattention and mood in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry 48, 1094–1101 (2009).
Craddock, N., O'Donovan, M.C. & Owen, M.J. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr. Bull. 32, 9–16 (2006).
Green, E.K. et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol. Psychiatry 15, 1016–1022 (2010).
Williams, N.M. et al. Genome-wide analysis of copy number variants in attention deficit/hyperactivity disorder confirms the role of rare variants and implicates duplications at 15q13.3. Am. J. Psychiatry 169, 195–204 (2012).
Manolio, T.A. Genomewide association studies and assessment of the risk of disease. N. Engl. J. Med. 363, 166–176 (2010).
Lee, S.H., Wray, N.R., Goddard, M.E. & Visscher, P.M. Estimating missing heritability for disease from genome-wide association studies. Am. J. Hum. Genet. 88, 294–305 (2011).
Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42, 565–569 (2010).
Lee, S.H., Yang, J., Goddard, M.E., Visscher, P.M. & Wray, N.R. Estimation of pleiotropy between complex diseases using SNP-derived genomic relationships and restricted maximum likelihood. Bioinformatics 28, 2540–2542 (2012).
Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 43, 969–976 (2011).
Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 43, 977–983 (2011).
Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry 18, 497–511 (2013).
Anney, R. et al. Individual common variants exert weak effects on the risk for autism spectrum disorderspi. Hum. Mol. Genet. 21, 4781–4792 (2012).
Cross-Disorder Group of the Psychiatric GWAS Consortium. Genome-wide analysis identifies loci with shared effects on five major psychiatric disorders. Lancet 381, 1371–1379 (2013).
Neale, B.M. et al. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 49, 884–897 (2010).
Stergiakouli, E. et al. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am. J. Psychiatry 169, 186–194 (2012).
Lionel, A.C. et al. Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci. Transl. Med. 3, 95ra75 (2011).
Hinney, A. et al. Genome-wide association study in German patients with attention deficit/hyperactivity disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 156B, 888–897 (2011).
Ribasés, M. et al. Exploration of 19 serotoninergic candidate genes in adults and children with attention-deficit/hyperactivity disorder identifies association for 5HT2A, DDC and MAOB. Mol. Psychiatry 14, 71–85 (2009).
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Sinauer Associates, Sunderland, MA, 1998).
Purcell, S.M. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009).
Lee, S.H., Goddard, M.E., Wray, N.R. & Visscher, P.M. A better coefficient of determination for genetic profile analysis. Genet. Epidemiol. 36, 214–224 (2012).
Raychaudhuri, S. et al. Accurately assessing the risk of schizophrenia conferred by rare copy-number variation affecting genes with brain function. PLoS Genet. 6, e1001097 (2010).
Lee, S.H. et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat. Genet. 44, 247–250 (2012).
Lubke, G.H. et al. Estimating the genetic variance of major depressive disorder due to all single nucleotide polymorphisms. Biol. Psychiatry 72, 707–709 (2012).
Klei, L. et al. Common genetic variants, acting additively, are a major source of risk of autism. Mol. Autism 3, 9 (2012).
Browning, S.R. & Browning, B.L. Population structure can inflate SNP-based heritability estimates. Am. J. Hum. Genet. 89, 191–193, author reply 193–195 (2011).
Yang, J. et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 43, 519–525 (2011).
Lee, S.H. et al. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer's disease, multiple sclerosis and endometriosis. Hum. Mol. Genet. 22, 832–841 (2013).
Constantino, J.N. & Todd, R.D. Intergenerational transmission of subthreshold autistic traits in the general population. Biol. Psychiatry 57, 655–660 (2005).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Loftus, E.V. Jr. et al. Increased risks of developing anxiety and depression in young patients with Crohn's disease. Am. J. Gastroenterol. 106, 1670–1677 (2011).
Kohane, I.S. et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS ONE 7, e33224 (2012).
Benach, J.L., Li, E. & McGovern, M.M. A microbial association with autism. mBio 3, e00019–12 (2012).
Wray, N.R., Lee, S.H. & Kendler, K.S. Impact of diagnostic misclassification on estimation of genetic correlations using genome-wide genotypes. Eur. J. Hum. Genet. 20, 668–674 (2012).
Bromet, E.J. et al. Diagnostic shifts during the decade following first admission for psychosis. Am. J. Psychiatry 168, 1186–1194 (2011).
Laursen, T.M., Agerbo, E. & Pedersen, C.B. Bipolar disorder, schizoaffective disorder, and schizophrenia overlap: a new comorbidity index. J. Clin. Psychiatry 70, 1432–1438 (2009).
Tsuang, M.T., Woolson, R.F., Winokur, G. & Crowe, R.R. Stability of psychiatric diagnosis. Schizophrenia and affective disorders followed up over a 30- to 40-year period. Arch. Gen. Psychiatry 38, 535–539 (1981).
Visscher, P.M., Brown, M.A., McCarthy, M.I. & Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 90, 7–24 (2012).
Wray, N.R. et al. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol. Psychiatry 17, 36–48 (2012).
Falconer, D. & Mackay, T. Introduction to Quantitative Genetics 4th edn. (Longman Scientific & Technical, Harlow, UK, 1996).
Van Snellenberg, J.X. & de Candia, T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch. Gen. Psychiatry 66, 748–755 (2009).
Lichtenstein, P. et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373, 234–239 (2009).
McGuffin, P. et al. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch. Gen. Psychiatry 60, 497–502 (2003).
Moreno-De-Luca, D. et al. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am. J. Hum. Genet. 87, 618–630 (2010).
Stankiewicz, P. & Lupski, J.R. Structural variation in the human genome and its role in disease. Annu. Rev. Med. 61, 437–455 (2010).
Nijmeijer, J.S. et al. Identifying loci for the overlap between attention-deficit/hyperactivity disorder and autism spectrum disorder using a genome-wide QTL linkage approach. J. Am. Acad. Child Adolesc. Psychiatry 49, 675–685 (2010).
Mulligan, A. et al. Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disorders. J. Autism Dev. Disord. 39, 197–209 (2009).
Ripke, S.A. et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. (in the press).
Sullivan, P.F., Daly, M.J. & O'Donovan, M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat. Rev. Genet. 13, 537–551 (2012).
Reich, T., James, J.W. & Morris, C.A. The use of multiple thresholds in determining the mode of transmission of semi-continuous traits. Ann. Hum. Genet. 36, 163–184 (1972).
Cochran, W.G. The combination of estimates from different experiments. Biometrics 10, 101–129 (1954).
Higgins, J.P., Thompson, S.G., Deeks, J.J. & Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 327, 557–560 (2003).
Yang, J., Lee, S.H., Goddard, M.E. & Visscher, P.M. GCTA: a tool for Genome-wide Complex Trait Analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Acknowledgements
This research was directly supported by the Australian Research Council (FT0991360 and DE130100614) and the Australian National Health and Medical Research Council (613608, 1011506 and 1047956). The PGC Cross-Disorder Group is supported by National Institute of Mental Health (NIMH) grant U01 MH085520. Statistical analyses were carried out on the Genetic Cluster Computer (see URLs), which is financially supported by the Netherlands Scientific Organization (NOW; 480-05-003; principal investigator D.P.) along with a supplement from the Dutch Brain Foundation and VU University. Numerous (>100) grants from government agencies along with substantial private and foundation support worldwide enabled the collection of phenotype and genotype data, without which this research would not be possible; grant numbers are listed in primary PGC publications or in the Supplementary Note.
Author information
Authors and Affiliations
Consortia
Contributions
Project conception: K.S.K., N.R.W. and J.W.S. Analysis: S.H.L. and N.R.W. Writing of the manuscript: N.R.W., S.H.L., K.S.K. and S.V.F. Quality control for PGC data: S. Ripke and B.M.N. Revisions to the manuscript: S.M.P., J.W.S., R.H.P., B.J.M., P.F.S., A.T., C.O., M.J.D., R.D.O. and J.B. Statistical advice: M.E.G. and J.S.W. Data access: D.P. PGC Workgroup Chairs: M.J.D. (analysis), S.V.F. (ADHD), M.J.D. and B.D. (co-chairs ASD), J.K. and P. Sklar (co-chairs bipolar disorder), P.F.S. (major depressive disorder), M.C.O. (schizophrenia) and J.W.S. and K.S.K. (co-chairs cross-disorder group). Collection, genotyping and analysis for PGC Working Groups. PGC ADHD Working Group: B.M.N., S.V.F., A.T., R.A., P.A., T. Banaschewski, M. Bayés, J.B., J.K.B., M.C., B.C., J.C., A.E.D., R.P.E., J.E., B.F., C.M.F., L. Kent, J.K., K.-P.L., S.K.L., J.M., J.J.M., S.E.M., J.M.S., A. Miranda, S.F.N., R.D.O., J.A.R.-Q., A. Reif, M. Ribasés, H.R., A. Rothenberger, J.A.S., R.S., S.L. Smalley, E.J.S.S.-B., H.-C.S., A.A.T. and N.W. PGC ASD Working Group: R.A., D.E.A., A.J.B., A.B., C.B., J.D. Buxbaum, A. Chakravarti, E.H.C., H.C., M.L.C., G.D., E.D., S.E., E.F., C.M.F., L. Gallagher, D.H.G., M. Gill, D.E.G., J.L.H., H.H., J.H., V.H., S.M.K., L. Klei, D.H. Ledbetter, C. Lord, J.K.L., E.M., S.M.M., C.L.M., W.M.M., A.P.M., D.M.-D.-L., E.M.M., M. Murtha, G.O., A.P., J.R.P., A.D.P., M.A.P.-V., J. Piven, F.P., K. Rehnström, K. Roeder, G.R., S.J.S., S.L. Santangelo, G.D.S., S.W.S., M. State, J.S. Sutcliffe, P. Szatmari, A.M.V., V.J.V., C.A.W., T.H.W., E.M.W., A.J.W., T.W.Y., B.D. and M.J.D. PGC BPD Working Group: S.M.P., D.A., H.A., O.A.A., A.A., L.B., J.A.B., J.D. Barchas, T.B.B., N.B., M. Bauer, F.B., S.E.B., W.B., D.H.R.B., C.S.B., M. Boehnke, G.B., R. Breuer, W.E.B., W.F.B., S. Caesar, K. Chambert, S. Cichon, D.A.C., A. Corvin, W.H.C., D.W.C., R.D., F. Degenhardt, S. Djurovic, F. Dudbridge, H.J.E., B.E., A.E.F., I.N.F., M. Flickinger, T.F., J.F., C.F., L.F., E.S.G., M. Gill, K.G.-S., E.K.G., T.A.G., D.G., W.G., H.G., M.L.H., M. Hautzinger, S. Herms, M. Hipolito, P.A.H., C.M.H., S.J., E.G.J., I.J., L.J., R. Kandaswamy, J.L.K., G.K.K., D.L.K., P.K., M. Landén, N.L., M. Lathrop, J. Lawrence, W.B.L., M. Leboyer, P.H.L., J. Li, P.L., D.-Y.L., C. Liu, F.W.L., S.L., P.B.M., W.M., N.G.M., M. Mattheisen, K.M., M. Mattingsdal, K.A.M., P.M., M.G.M., A. McIntosh, R.M., A.W.M., F.J.M., A. McQuillin, S.M., I.M., F.M., G.W.M., J.L.M., G.M., D.W.M., V. Moskvina, P.M., T.W.M., W.J.M., B.M.-M., R.M.M., C.M.N., I.N., V.N., M.M.N., J.I.N., E.A.N., C.O., U.O., M.J.O., B.S.P., J.B.P., P.P., E.M.Q., S. Raychaudhuri, A. Reif, J.P.R., M. Rietschel, D. Ruderfer, M. Schalling, A.F.S., W.A.S., N.J.S., T.G.S., J. Schumacher, M. Schwarz, E.S., L.J.S., P.D.S., E.N.S., D.S.C., M. Steffens, J.S. Strauss, J. Strohmaier, S.S., R.C.T., F.T., J.T., J.B.V., S.J.W., T.F.W., S.H.W., W.X., A.H.Y., P.P.Z., P.Z., S. Zöllner, J.R.K., P. Sklar, M.J.D., M.C.O. and N.C. PGC MDD Working Group: M.R.B., T. Bettecken, E.B.B., D.H.R.B., D.I.B., G.B., R. Breuer, S. Cichon, W.H.C., I.W.C., D. Czamara, E.J.D.G., F. Degenhardt, A.E.F., J.F., S.D.G., M. Gross, S.P.H., A.C.H., A.K.H., S. Herms, I.B.H., F.H., W.J.H., S. Hoefels, J.-J.H., M.I., I.J., L.J., J.-Y. T., J.A.K., M.A.K., A.K., W.B.L., D.F.L., C.M.L., D.-Y.L., S.L., D.J.M., P.A.F.M., W.M.,. N.G.M., M. Mattheisen, P.J.M., P.M., A. McIntosh, A.W.M., C.M.M., L.M., G.W.M., P.M., B.M.-M., W.A.N., M.M.N., D.R.N., B.W.P., M.L.P., J.B.P., M. Rietschel, W.A.S., T.G.S., J. Shi, S.I.S., S.L. Slager, J.H.S., M. Steffens, F.T., J.T., M.U., E.J.C.G.v.d.O., G.V.G., M.M.W., G.W., F.G.Z., P.F.S. and N.R.W. PGC SCZ Working Group: S. Ripke, B.M.N., S.M.P., B.J.M., I.A., F.A., O.A.A., M.H.A., N.B., D.W.B., D.H.R.B., R. Bruggeman, N.G.B., W.F.B., W.C., R.M.C., K. Choudhury, S. Cichon, C.R.C., P.C., A. Corvin, D. Curtis, S. Datta, S. Djurovic, G.J.D., J.D., F. Dudbridge, A.F., R.F., N.B.F., M. Friedl, P.V.G., L. Georgieva, I.G., M. Gill, H.G., L.D.H., M.L.H., T.F.H., A.M.H., P.A.H., C.M.H., A.I., A.K.K, R.S.K., M.C.K., E.K., Y.K., G.K.K., B.K., L. Krabbendam, R. Krasucki, J. Lawrence, P.H.L., T.L., D.F.L., J.A.L., D.-Y.L., D.H. Linszen, P.K.E.M., W.M., A.K.M., M. Mattheisen, M. Mattingsdal, S.M., S.A.M., A. McIntosh, A. McQuillin, H.M., I.M., V. Milanova, D.W.M., V. Moskvina, I.M.-G., M.M.N., C.O., A.O., L.O., R.A.O., M.J.O., C.N.P., M.T.P., B.S.P., J. Pimm, D.P., V.P., D.J.Q., H.B.R., M. Rietschel, L.R., D. Ruderfer, D. Rujescu, A.R.S., T.G.S., J. Shi, J.M.S., D.S.C., T.S.S., S.T., J.V.O., P.M.V., T.W., S. Zammit, P. Sklar, M.J.D., M.C.O., N.C., P.F.S. and K.S.K. PGC Cross-Disorder Group Working Group: S.H.L., S. Ripke, B.M.N., S.M.P., R.H.P., A.T., A.F., M.C.N., J.I.N., B.W.P., M. Rietschel, T.G.S., N.C., S.L. Santangelo, P.F.S., J.W.S., K.S.K. and N.R.W. PGC Analysis Working Group: S.H.L., S. Ripke, B.M.N., S.M.P., V.A., E.M.B., P.H.L., S.E.M., M.C.N., D.P., M.J.D. and N.R.W.
Corresponding author
Ethics declarations
Competing interests
The author declare no competing financial interests.
Additional information
A list of members appears in the Supplementary Note.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–9, Supplementary Figures 1 and 2 and Supplementary Note (PDF 960 kb)
Rights and permissions
About this article
Cite this article
Cross-Disorder Group of the Psychiatric Genomics Consortium. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 45, 984–994 (2013). https://doi.org/10.1038/ng.2711
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2711
This article is cited by
-
Rare genetic brain disorders with overlapping neurological and psychiatric phenotypes
Nature Reviews Neurology (2024)
-
Induction of dopaminergic neurons for neuronal subtype-specific modeling of psychiatric disease risk
Molecular Psychiatry (2023)
-
Clinical characteristics indexing genetic differences in bipolar disorder – a systematic review
Molecular Psychiatry (2023)
-
Disrupted extracellular matrix and cell cycle genes in autism-associated Shank3 deficiency are targeted by lithium
Molecular Psychiatry (2023)
-
Identifying the mediating role of socioeconomic status on the relationship between schizophrenia and major depressive disorder: a Mendelian randomisation analysis
Schizophrenia (2023)