Abstract
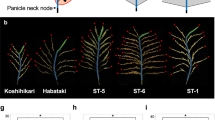
Reduction in seed shattering was an important phenotypic change during cereal domestication1,2. Here we show that a simple morphological change in rice panicle shape, controlled by the SPR3 locus, has a large impact on seed-shedding and pollinating behaviors. In the wild genetic background of rice, we found that plants with a cultivated-like type of closed panicle had significantly reduced seed shedding through seed retention. In addition, the long awns in closed panicles disturbed the free exposure of anthers and stigmas on the flowering spikelets, resulting in a significant reduction of the outcrossing rate. We localized the SPR3 locus to a 9.3-kb genomic region, and our complementation tests suggest that this region regulates the liguleless gene (OsLG1). Sequencing analysis identified reduced nucleotide diversity and a selective sweep at the SPR3 locus in cultivated rice. Our results suggest that a closed panicle was a selected trait during rice domestication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Harlan, J.R., de Wet, J.M. & Price, E.G. Comparative evolution of cereals. Evolution 27, 311–325 (1973).
Flannery, K.V. The origins of agriculture. Annu. Rev. Anthropol. 2, 271–310 (1973).
Khush, G.S. Origin, dispersal, cultivation and variation of rice. Plant Mol. Biol. 35, 25–34 (1997).
Oka, H.I. Origin of Cultivated Rice (Elsevier, Amsterdam, 1988).
Mannion, A.M. Domestication and the origins of agriculture: an appraisal. Prog. Phys. Geogr. 23, 37–56 (1999).
Konishi, S. et al. An SNP caused loss of seed shattering during rice domestication. Science 312, 1392–1396 (2006).
Li, C., Zhou, A. & Sang, T. Genetic analysis of rice domestication syndrome with the wild annual species, Oryza nivara. New Phytol. 170, 185–193 (2006).
Li, C., Zhou, A. & Sang, T. Rice domestication by reducing shattering. Science 311, 1936–1939 (2006).
Ishikawa, R. et al. Allelic interaction at seed-shattering loci in the genetic backgrounds of wild and cultivated rice species. Genes Genet. Syst. 85, 265–271 (2010).
Thurber, C.S. et al. Molecular evolution of shattering loci in U.S. weedy rice. Mol. Ecol. 19, 3271–3284 (2010).
Eiguchi, M. & Sano, Y. A gene complex responsible for seed shattering and panicle spreading found in common wild rices. Rice Genet. Newsletter 7, 105–107 (1990).
Luo, J.J., Hao, W., Jin, J., Gao, J.P. & Lin, H.X. Fine mapping of Spr3, a locus for spreading panicle from African cultivated rice (Oryza glaberrima Steud.). Mol. Plant 1, 830–838 (2008).
Lee, J., Park, J.J., Kim, S.L., Yim, J. & An, G. Mutations in the rice liguleless gene result in a complete loss of the auricle, ligule, and laminar joint. Plant Mol. Biol. 65, 487–499 (2007).
Clark, R.M., Linton, E., Messing, J. & Doebley, J.F. Pattern of diversity in the genomic region near the maize domestication gene tb1. Proc. Natl. Acad. Sci. USA 101, 700–707 (2004).
Purugganan, M.D. & Fuller, D.Q. The nature of selection during plant domestication. Nature 457, 843–848 (2009).
Thanh, P.T., Phan, P.D.T., Mori, N., Ishikawa, R. & Ishii, T. Development of backcross recombinant inbred lines between Oryza sativa Nipponbare and O. rufipogon and QTL detection on drought tolerance. Breed. Sci. 61, 76–79 (2011).
IRRI. Standard evaluation system for rice (International Rice Research Institute, Philippines, 2002).
McCouch, S.R. et al. Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res. 9, 257–279 (2002).
Nelson, J.C. qGENE: Software for marker-based genomic analysis and breeding. Mol. Breed. 3, 239–245 (1997).
Vaughan, D.A., Balazs, E. & Heslop-Harrison, J.S. From crop domestication to super-domestication. Ann. Bot. 100, 893–901 (2007).
Librado, P. & Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Londo, J.P., Chiang, Y.C., Hung, K.H., Chiang, T.Y. & Shaal, B.A. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc. Natl. Acad. Sci. USA 103, 9578–9583 (2006).
Zhu, Q., Zheng, X., Luo, J., Gaut, B.S. & Ge, S. Multilocus analysis of nucleotide variation of Oryza sativa and its wild relatives: severe bottleneck during domestication of rice. Mol. Biol. Evol. 24, 875–888 (2007).
Wright, S.I. & Charlesworth, B. The HKA test revisited: a maximum-likelihood-ratio test of the standard neutral model. Genetics 168, 1071–1076 (2004).
Gao, L.Z. & Innan, H. Nonindependent domestication of the two rice subspecies, Oryza sativa ssp. indica and ssp. japonica, demonstrated by multilocus microsatellites. Genetics 179, 965–976 (2008).
Asano, K. et al. Artificial selection for a green revolution gene during japonica rice domestication. Proc. Natl. Acad. Sci. USA 108, 11034–11039 (2011).
Acknowledgements
We thank the National Institute of Genetics (National Bioresource Project), Japan, and the National Institute of Agrobiological Sciences, Japan, for providing the seeds of wild and cultivated rice, Y. Takezaki and P.D.T. Phuong for helping with field experiments and H. Fukaki for supporting expression analysis. This work was supported in part by a Grant-in-Aid from Japanese Society for Promotion of Science to T.I. (20580005, 23580006, 23.01390) and by the Japan Science and Technology Agency-Japan International Cooperation Agency within the framework of the Science and Technology Research Partnership for Sustainable Development to M.A.
Author information
Authors and Affiliations
Contributions
T.I., K.N. and P.T.T. performed the field experiments and analyzed the results. T.M.H. and R.I. conducted the histological analysis. T.M. produced constructs, and K.M., N.K., R.I. and M.A. generated and analyzed transformants. K.N., M.Y., K.Y. and R.T. participated in sequence analysis, and T.I. and M.A. designed the research and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Tables 13 (PDF 6060 kb)
Rights and permissions
About this article
Cite this article
Ishii, T., Numaguchi, K., Miura, K. et al. OsLG1 regulates a closed panicle trait in domesticated rice. Nat Genet 45, 462–465 (2013). https://doi.org/10.1038/ng.2567
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2567
This article is cited by
-
A mediator of OsbZIP46 deactivation and degradation negatively regulates seed dormancy in rice
Nature Communications (2024)
-
Genome-wide identification and analysis of SPL gene family in chickpea (Cicer arietinum L.)
Protoplasma (2024)
-
Can the Wild Perennial, Rhizomatous Rice Species Oryza longistaminata be a Candidate for De Novo Domestication?
Rice (2023)
-
SPR9 encodes a 60 S ribosomal protein that modulates panicle spreading and affects resistance to false smut in rice (Oryza sativa. L)
BMC Plant Biology (2023)
-
Substitution Mapping and Allelic Variations of the Domestication Genes from O. rufipogon and O. nivara
Rice (2023)