Abstract
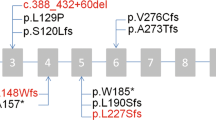
Dystonia is a movement disorder characterized by repetitive twisting muscle contractions and postures1,2. Its molecular pathophysiology is poorly understood, in part owing to limited knowledge of the genetic basis of the disorder. Only three genes for primary torsion dystonia (PTD), TOR1A (DYT1)3, THAP1 (DYT6)4 and CIZ1 (ref. 5), have been identified. Using exome sequencing in two families with PTD, we identified a new causative gene, GNAL, with a nonsense mutation encoding p.Ser293* resulting in a premature stop codon in one family and a missense mutation encoding p.Val137Met in the other. Screening of GNAL in 39 families with PTD identified 6 additional new mutations in this gene. Impaired function of several of the mutants was shown by bioluminescence resonance energy transfer (BRET) assays.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Fahn, S. Concept and classification of dystonia. Adv. Neurol. 50, 1–8 (1988).
Fahn, S., Bressman, S.B. & Marsden, C.D. Classification of dystonia. Adv. Neurol. 78, 1–10 (1998).
Ozelius, L.J. et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat. Genet. 17, 40–48 (1997).
Fuchs, T. et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat. Genet. 41, 286–288 (2009).
Xiao, J. et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann. Neurol. 71, 458–469 (2012).
Albanese, A. et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur. J. Neurol. 18, 5–18 (2011).
Ozelius, L.J. & Bressman, S.B. Genetic and clinical features of primary torsion dystonia. Neurobiol. Dis. 42, 127–135 (2011).
Risch, N.J. et al. Segregation analysis of idiopathic torsion dystonia in Ashkenazi Jews suggests autosomal dominant inheritance. Am. J. Hum. Genet. 46, 533–538 (1990).
Ahmad, F. et al. Evidence for locus heterogeneity in autosomal dominant torsion dystonia. Genomics 15, 9–12 (1993).
Defazio, G., Livrea, P., Guanti, G., Lepore, V. & Ferrari, E. Genetic contribution to idiopathic adult-onset blepharospasm and cranial-cervical dystonia. Eur. Neurol. 33, 345–350 (1993).
Bressman, S.B. et al. Idiopathic dystonia among Ashkenazi Jews: evidence for autosomal dominant inheritance. Ann. Neurol. 26, 612–620 (1989).
Waddy, H.M., Fletcher, N.A., Harding, A.E. & Marsden, C.D. A genetic study of idiopathic focal dystonias. Ann. Neurol. 29, 320–324 (1991).
Bressman, S.B. et al. A study of idiopathic torsion dystonia in a non-Jewish family: evidence for genetic heterogeneity. Neurology 44, 283–287 (1994).
Bressman, S.B. et al. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 8, 441–446 (2009).
Saunders-Pullman, R. et al. Variant ataxia-telangiectasia presenting as primary-appearing dystonia in Canadian Mennonites. Neurology 78, 649–657 (2012).
Kumar, P., Henikoff, S. & Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Sheth, N. et al. Comprehensive splice-site analysis using comparative genomics. Nucleic Acids Res. 34, 3955–3967 (2006).
Blanchard, A. et al. DYT6 dystonia: review of the literature and creation of the UMD Locus-Specific Database (LSDB) for mutations in the THAP1 gene. Hum. Mutat. 32, 1213–1224 (2011).
Corradi, J.P. et al. Alternative transcripts and evidence of imprinting of GNAL on 18p11.2. Mol. Psychiatry 10, 1017–1025 (2005).
Leube, B. et al. Idiopathic torsion dystonia: assignment of a gene to chromosome 18p in a German family with adult onset, autosomal dominant inheritance and purely focal distribution. Hum. Mol. Genet. 5, 1673–1677 (1996).
Grimes, D.A. et al. A novel locus for inherited myoclonus-dystonia on 18p11. Neurology 59, 1183–1186 (2002).
Han, F., Racacho, L., Lang, A.E., Bulman, D.E. & Grimes, D.A. Refinement of the DYT15 locus in myoclonus dystonia. Mov. Disord. 22, 888–892 (2007).
Tezzon, F., Zanoni, T., Passarin, M.G. & Ferrari, G. Dystonia in a patient with deletion of 18p. Ital. J. Neurol. Sci. 19, 90–93 (1998).
Klein, C. et al. Genetic analysis of three patients with an 18p– syndrome and dystonia. Neurology 52, 649–651 (1999).
Nasir, J. et al. Unbalanced whole arm translocation resulting in loss of 18p in dystonia. Mov. Disord. 21, 859–863 (2006).
Postma, A.G., Verschuuren-Bemelmans, C.C., Kok, K. & van Laar, T. Characteristics of dystonia in the 18p deletion syndrome, including a new case. Clin. Neurol. Neurosurg. 111, 880–882 (2009).
Graziadio, C. et al. Dystonia, autoimmune disease and cerebral white matter abnormalities in a patient with 18p deletion. Arq. Neuropsiquiatr. 67, 689–691 (2009).
Kowarik, M.C. et al. Myoclonus-dystonia in 18p deletion syndrome. Mov. Disord. 26, 560–561 (2011).
Jones, D.T. & Reed, R.R. Golf: an olfactory neuron specific–G protein involved in odorant signal transduction. Science 244, 790–795 (1989).
Oldham, W.M. & Hamm, H.E. Heterotrimeric G protein activation by G-protein–coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60–71 (2008).
Drinnan, S.L., Hope, B.T., Snutch, T.P. & Vincent, S.R. Golf in the basal ganglia. Mol. Cell. Neurosci. 2, 66–70 (1991).
Hervé, D. et al. Golf and Gs in rat basal ganglia: possible involvement of Golf in the coupling of dopamine D1 receptor with adenylyl cyclase. J. Neurosci. 13, 2237–2248 (1993).
Kull, B., Svenningsson, P. & Fredholm, B.B. Adenosine A2A receptors are colocalized with and activate Golf in rat striatum. Mol. Pharmacol. 58, 771–777 (2000).
Hervé, D. et al. Gαolf levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J. Neurosci. 21, 4390–4399 (2001).
Corvol, J.C., Studler, J.M., Schonn, J.S., Girault, J.A. & Herve, D. Gαolf is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J. Neurochem. 76, 1585–1588 (2001).
Hervé, D. Identification of a specific assembly of the G protein Golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front. Neuroanat. 5, 48 (2011).
Sunahara, R.K., Tesmer, J.J., Gilman, A.G. & Sprang, S.R. Crystal structure of the adenylyl cyclase activator Gsα . Science 278, 1943–1947 (1997).
Berardelli, A. et al. The pathophysiology of primary dystonia. Brain 121, 1195–1212 (1998).
Breakefield, X.O. et al. The pathophysiological basis of dystonias. Nat. Rev. Neurosci. 9, 222–234 (2008).
Neychev, V.K., Gross, R.E., Lehericy, S., Hess, E.J. & Jinnah, H.A. The functional neuroanatomy of dystonia. Neurobiol. Dis. 42, 185–201 (2011).
Page, M.E. et al. Cell-autonomous alteration of dopaminergic transmission by wild type and mutant (ΔE) TorsinA in transgenic mice. Neurobiol. Dis. 39, 318–326 (2010).
Sciamanna, G. et al. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiol. Dis. 34, 133–145 (2009).
Bonsi, P. et al. Centrality of striatal cholinergic transmission in basal ganglia function. Front. Neuroanat. 5, 6 (2011).
Corvol, J.C. et al. Quantitative changes in Gαolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants. Neuropsychopharmacology 32, 1109–1121 (2007).
Belluscio, L., Gold, G.H., Nemes, A. & Axel, R. Mice deficient in Golf are anosmic. Neuron 20, 69–81 (1998).
Zhuang, X., Belluscio, L. & Hen, R. Golfα mediates dopamine D1 receptor signaling. J. Neurosci. 20, RC91 (2000).
Corvol, J.C. et al. Persistent increase in olfactory type G-protein α subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J. Neurosci. 24, 7007–7014 (2004).
Alcacer, C. et al. Gαolf mutation allows parsing the role of cAMP-dependent and extracellular signal–regulated kinase–dependent signaling in L-3,4-dihydroxyphenylalanine–induced dyskinesia. J. Neurosci. 32, 5900–5910 (2012).
Del Sorbo, F. & Albanese, A. Levodopa-induced dyskinesias and their management. J. Neurol. 255 (suppl. 4), 32–41 (2008).
Balcioglu, A. et al. Dopamine release is impaired in a mouse model of DYT1 dystonia. J. Neurochem. 102, 783–788 (2007).
Sako, W., Morigaki, R., Nagahiro, S., Kaji, R. & Goto, S. Olfactory type G-protein α subunit in striosome-matrix dopamine systems in adult mice. Neuroscience 170, 497–502 (2010).
Crittenden, J.R. & Graybiel, A.M. Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front. Neuroanat. 5, 59 (2011).
Kamnasaran, D. Genetic analysis of psychiatric disorders associated with human chromosome 18. Clin. Invest. Med. 26, 285–302 (2003).
Laurin, N. et al. Investigation of the G protein subunit Gαolf gene (GNAL) in attention deficit/hyperactivity disorder. J. Psychiatr. Res. 42, 117–124 (2008).
DasBanerjee, T. et al. A comparison of molecular alterations in environmental and genetic rat models of ADHD: a pilot study. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 147B, 1554–1563 (2008).
Ozelius, L.J., Lubarr, N. & Bressman, S.B. Milestones in dystonia. Mov. Disord. 26, 1106–1126 (2011).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Jarman, P.R. et al. Primary torsion dystonia: the search for genes is not over. J. Neurol. Neurosurg. Psychiatry 67, 395–397 (1999).
Factor, S.A. Cervical dystonia in twins. Mov. Disord. 17, 846–847 (2002).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Dayem Ullah, A.Z., Lemoine, N.R. & Chelala, C. SNPnexus: a web server for functional annotation of novel and publicly known genetic variants (2012 update). Nucleic Acids Res. 40, W65–W70 (2012).
Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386 (2000).
Hollins, B., Kuravi, S., Digby, G.J. & Lambert, N.A. The C-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell. Signal. 21, 1015–1021 (2009).
Gopalakrishna, K.N. et al. Interaction of transducin with uncoordinated 119 protein (UNC119): implications for the model of transducin trafficking in rod photoreceptors. J. Biol. Chem. 286, 28954–28962 (2011).
Niznik, H.B. et al. Dopamine D1 receptors characterized with [3H]SCH 23390. Solubilization of a guanine nucleotide–sensitive form of the receptor. J. Biol. Chem. 261, 8397–8406 (1986).
Acknowledgements
We wish to thank all individuals with dystonia and their family members who participated in this study. We are indebted to the following physicians for referring families with dystonia included in this manuscript: S. Reich, J. Hammerstadt, D. Hobson, D. Truong, F. Danisi, M. Hutchinson, S. O'Riordan, T. Lynch and J. Rogers. We would also like to thank the following physicians who examined study subjects during their movement disorder fellowships: R. Tabamo, E. Chai, K. Blatt, P. Kavanagh, P. Kapoor and G. Petzinger. We thank R. Sachidanandam for the bioinformatics analysis related to the splice-site mutation; H. Lederman for technical help; N.A. Lambert (Georgia Health Sciences University) for sharing Venus155-239-Gβ1 and Venus1-155-Gγ2; and B. Malnic (Universidade de São Paulo) for the gift of the Ric-8B construct. This work was supported by research grants from the Dystonia Medical Research Foundation (T.F.), the Bachmann-Strauss Dystonia and Parkinson Foundation (L.J.O.), the Lockwood Family Foundation (N.S. and L.J.O.), the National Institute of Neurological Disorders and Stroke (NS26656, S.B.B., R.S.-P. and L.J.O.; NS037409, N.S. and L.J.O.; K02-NS073836, R.S.-P.) the National Institute on Drug Abuse (DA021743 and DA026405, K.A.M.) and Agence Nationale de la Recherche (ANR09-MNPS-014, D.H.). The authors would like to thank the NHLBI GO Exome Sequencing Project and its ongoing studies, which produced and provided exome variant calls for comparison, including the Lung GO Sequencing Project (HL-102923), the Women's Health Initiative (WHI) Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
Author information
Authors and Affiliations
Contributions
T.F. developed the computational analysis pipeline, analyzed the next-generation and Sanger sequencing data and wrote the manuscript. S.W. and E.A. performed molecular experiments. N.S. provided funding for the linkage studies. D.H. provided the antibody to GNAL. D.R. collected samples and provided clinical information for the subjects. M.S.L. and R.S.-P. performed the statistical analysis related to phenotype. S.F., A.E.L., T.-W.L., R.M.T., R.S.-P. and S.B.B. examined subjects. S.B.B. and R.S.-P. supervised the acquisition of clinical data and blood samples and assigned final clinical status. K.A.M. and M.E.E. formulated the functional assay. I.M. performed the assays of Gαolf function. K.A.M. and I.M. analyzed and interpreted the functional data. L.J.O. designed and supervised the genetic studies. T.F., I.M., A.E.L., D.H., D.R., R.S.-P., M.S.L., N.S., K.A.M., M.E.E., S.B.B. and L.J.O. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Tables 1–5 and Supplementary Note (PDF 372 kb)
Rights and permissions
About this article
Cite this article
Fuchs, T., Saunders-Pullman, R., Masuho, I. et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet 45, 88–92 (2013). https://doi.org/10.1038/ng.2496
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2496
This article is cited by
-
Rare genetic brain disorders with overlapping neurological and psychiatric phenotypes
Nature Reviews Neurology (2024)
-
Structural brain heterogeneity underlying symptomatic and asymptomatic genetic dystonia: a multimodal MRI study
Journal of Neurology (2024)
-
Sensory processing in the auditory and olfactory domains is normal in laryngeal dystonia
Journal of Neurology (2023)
-
Genetic screening in patients of Meige syndrome and blepharospasm
Neurological Sciences (2022)
-
Ackr3-Venus knock-in mouse lights up brain vasculature
Molecular Brain (2021)