Abstract
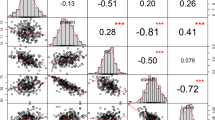
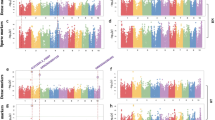
Maize kernel oil is a valuable source of nutrition. Here we extensively examine the genetic architecture of maize oil biosynthesis in a genome-wide association study using 1.03 million SNPs characterized in 368 maize inbred lines, including 'high-oil' lines. We identified 74 loci significantly associated with kernel oil concentration and fatty acid composition (P < 1.8 × 10−6), which we subsequently examined using expression quantitative trait loci (QTL) mapping, linkage mapping and coexpression analysis. More than half of the identified loci localized in mapped QTL intervals, and one-third of the candidate genes were annotated as enzymes in the oil metabolic pathway. The 26 loci associated with oil concentration could explain up to 83% of the phenotypic variation using a simple additive model. Our results provide insights into the genetic basis of oil biosynthesis in maize kernels and may facilitate marker-based breeding for oil quantity and quality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Thelen, J.J. & Ohlrogge, J.B. Metabolic engineering of fatty acid biosynthesis in plants. Metab. Eng. 4, 12–21 (2002).
Graham, I.A. & Eastmond, P.J. Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog. Lipid Res. 41, 156–181 (2002).
Beisson, F. et al. Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol. 132, 681–697 (2003).
Baud, S. & Lepiniec, L. Physiological and developmental regulation of seed oil production. Prog. Lipid Res. 49, 235–249 (2010).
Dudley, J.W. & Lambert, R.J. 100 generation of selection for oil and protein in corn. Plant Breed. Rev. 24, 79–110 (2004).
Lambert, R.J., Alexander, D.E. & Mejaya, I.J. Single kernel selection for increased grain oil in maize synthetics and high-oil hybrid development. Plant Breed. Rev. 24, 153–175 (2004).
Song, T.M. & Chen, S.J. Long term selection for oil concentration in five maize populations. Maydica 49, 9–14 (2004).
Laurie, C.C. et al. The genetic architecture of response to long-term artificial selection for oil concentration in the maize kernel. Genetics 168, 2141–2155 (2004).
Yang, X.H. et al. Major and minor QTL and epistasis contribute to fatty acid compositions and oil concentration in high-oil maize. Theor. Appl. Genet. 120, 665–678 (2010).
Cook, J.P. et al. Genetic Architecture of Maize Kernel Composition in the Nested Association Mapping and Inbred Association Panels. Plant Physiol. 158, 824–834 (2012).
Yan, J.B., Warburton, M. & Crouch, J. Association mapping for enhancing maize genetic improvement. Crop Sci. 51, 433–449 (2011).
Myles, S. et al. Association mapping: critical considerations shift from genotyping to experimental design. Plant Cell 21, 2194–2202 (2009).
Majewski, J. & Pastinen, T. The study of eQTL variations by RNA-seq: from SNPs to phenotypes. Trends Genet. 27, 72–79 (2011).
Ganal, M.W. et al. A large maize (Zea mays L.) SNP genotyping array: development and germplasm genotyping, and genetic mapping to compare with the B73 reference genome. PLoS ONE 6, e28334 (2011).
Yang, X.H. et al. Characterization of a global germplasm collection and its potential utilization for analysis of complex quantitative traits in maize. Mol. Breed. 28, 511–526 (2011).
Yu, J.M. et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38, 203–208 (2006).
Zhang, Z.W. et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 42, 355–360 (2010).
Buckler, E.S. et al. The genetic architecture of maize flowering time. Science 325, 714–718 (2009).
Tian, F. et al. Genome-wide association study of maize identifies genes affecting leaf architecture. Nat. Genet. 43, 159–162 (2011).
Kump, K.L. et al. Genome-wide association study of quantitative resistance to southern leaf blight in the maize nested association mapping population. Nat. Genet. 43, 163–168 (2011).
Poland, J.A., Bradbury, P.J., Buckler, E.S. & Nelson, R.J. Genome-wide nested association mapping of quantitative resistance to northern leaf blight in maize. Proc. Natl. Acad. Sci. USA 108, 6893–6898 (2011).
Beló, A. et al. Whole genome scan detects an allelic variant of fad2 associated with increased oleic acid levels in maize. Mol. Genet. Genomics 279, 1–10 (2008).
Zheng, P.Z. et al. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat. Genet. 40, 367–372 (2008).
Li, L. et al. An 11-bp insertion in Zea mays fatb reduces the palmitic acid content of fatty acids in maize grain. PLoS ONE 6, e24699 (2011).
Hurley, J.H. & Misra, S. Signaling and subcellular targeting by membrane-binding domains. Annu. Rev. Biophys. Biomol. Struct. 29, 49–79 (2000).
Yang, X.H. et al. Genetic analysis and characterization of a new maize association mapping panel for quantitative trait loci dissection. Theor. Appl. Genet. 121, 417–431 (2010).
Shintani, D.K. & Ohlrogge, J.B. The characterization of a mitochondrial acyl carrier protein isoform isolated from Arabidopsis thaliana. Plant Physiol. 104, 1221–1229 (1994).
Sanchez, J., Agrawal, V.P. & Stumpf, P.K. Structure, Function and Metabolism of Plant Lipids (eds. Siegenthaler, P.A. & Eichenberger, W.) (Elsevier, 1984).
Branen, J.K., Chiou, T.J. & Engeseth, N.J. Overexpression of acyl carrier protein-1 alters fatty acid composition of leaf tissue in Arabidopsis. Plant Physiol. 127, 222–229 (2001).
Li-Beisson, Y.H. et al. Acyl-lipid metabolism (eds. Somerville, C.R. & Meyerowitz, E.M.) The Arabidopsis Book, vol. 1 (American Society of Plant Biologists, 2010).
Cernac, A. & Benning, C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 40, 575–585 (2004).
Shen, B. et al. Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol. 153, 980–987 (2010).
Pouvreau, B. et al. Duplicate maize Wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol. 156, 674–686 (2011).
Gore, M.A. et al. A first-generation haplotype map of maize. Science 326, 1115–1117 (2009).
Huang, X.H. et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 42, 961–967 (2010).
Atwell, S. et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465, 627–631 (2010).
Zhang, Z.W., Buckler, E.S., Casstevens, T.M. & Bradbury, P.J. Software engineering the mixed model for genome-wide associated studies on large samples. Brief. Bioinform. 10, 664–675 (2009).
Platt, A., Vilhjálmsson, B.J. & Nordborg, M. Conditions under which genome-wide association studies will be positively misleading. Genetics 186, 1045–1052 (2010).
McClintock, B. The significance of responses of the genome to challenge. Science 226, 792–801 (1984).
Harjes, C.E. et al. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 319, 330–333 (2008).
Yan, J.B. et al. Rare genetic variation at Zea mays crtRB1 increases β-carotene in maize grain. Nat. Genet. 42, 322–327 (2010).
Studer, A., Zhao, Q., Ross-Ibarra, J. & Doebley, J. Identification of a functional transposon insertion in the maize domestication gene tb1. Nat. Genet. 43, 1160–1163 (2011).
Zhou, L.L., Zhang, J.Y., Yan, J.B. & Song, R.T. Two transposable element insertions are causative mutations for the major domestication gene teosinte branched 1 in modern maize. Cell Res. 21, 1267–1270 (2011).
Lal, S.K., Giroux, M.J., Brendel, V., Vallejos, C.E. & Hannah, L.C. The maize genome contains a helitron insertion. Plant Cell 15, 381–391 (2003).
Gupta, S. et al. A novel class of Helitron- related transposable elements in maize contain portions of multiple pseudogenes. Plant Mol. Biol. 57, 115–127 (2005).
Moose, S.P., Dudley, J.W. & Rocheford, T.R. Maize selection passes the century mark: a unique resource for 21st century genomics. Trends Plant Sci. 9, 358–364 (2004).
Chai, Y.C. et al. Validation of DGAT1–2 polymorphisms associated with oil content and development of functional markers for molecular breeding of high-oil maize. Mol. Breed. 29, 939–949 (2011).
Song, X.F., Song, T.M., Dai, J.R. & Rocheford, T.R. QTL mapping of kernel oil concentration with high–oil maize by SSR markers. Maydica 49, 41–48 (2004).
Mangolin, C.A. et al. Mapping QTLs for kernel oil content in a tropical maize population. Euphytica 137, 251–259 (2004).
Zhang, J. et al. Mapping quantitative trait loci for oil, starch, and protein concentrations in grain with high–oil maize by SSR markers. Euphytica 162, 335–344 (2008).
Newman, J.W., Morisseau, C. & Hammock, B.D. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog. Lipid Res. 44, 1–51 (2004).
Nawrath, C. The biopolymers cutin and suberin, vol. 2 (eds. Somerville, C.R. & Meyerowitz, E.M.) The Arabidopsis Book (American Society of Plant Biologists, 2010).
Safford, R. et al. Plastid–localised seed acyl–carrier protein of Brassica napus is encoded by a distinct, nuclear multigene family. FEBS J. 174, 287–295 (1988).
Evans, D.E., Taylor, P.E., Singh, M.B. & Knox, R.B. The interrelationship between the accumulation of lipids, protein and the level of acyl carrier protein during the development of Brassica napus L. pollen. Planta. 186, 343–354 (1992).
Kurz, E.U. & Lees-Miller, S.P. DNA damage–induced activation of ATM and ATM–dependent signaling pathways. DNA Repair 3, 889–900 (2004).
Shockey, J.M., Fulda, M.S. & Browse, J.A. Arabidopsis contains nine long–chain acyl–coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol. 129, 1710–1722 (2002).
Fulda, M., Shockey, J., Werber, M., Wolter, F.P. & Heinz, E. Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid beta-oxidation. Plant J. 32, 93–103 (2002).
Hellyer, S.A., Chandler, I.C. & Bosley, J.A. Can the fatty acid selectivity of plant lipases be predicted from the composition of the seed triglyceride? Biochim. Biophys. Acta 1440, 215–224 (1999).
Rosnitschek, I. & Theimer, R.R. Properties of a membrane–bound triglyceride lipase of rapeseed (Brassica napus L.) cotyledons. Planta 148, 193–198 (1980).
Paloccia, C. et al. Lipolytic isoenzymes from Euphorbia latex. Plant Sci. 165, 577–582 (2003).
Benveniste, I. et al. CYP86A1 from Arabidopsis thaliana encodes a cytochrome P450–dependent fatty acid omega–hydroxylase. Biochem. Bioph. Res. Co. 243, 688–693 (1998).
Song, W.C., Funk, C.D. & Brash, A.R. Molecular cloning of an allene oxide synthase: a cytochrome P450 specialized for the metabolism of fatty acid hydroperoxides. Proc. Natl. Acad. Sci. USA 90, 8519–8523 (1993).
Murata, N. & Tasaka, Y. Glycerol-3-phosphate acyltransferase in plants. Biochim. Biophys. Acta 1348, 10–16 (1997).
Yang, W.L. et al. A distinct type of glycerol–3–phosphate acyltransferase with sn–2 preference and phosphatase activity producing 2–monoacylglycerol. Proc. Natl. Acad. Sci. USA 107, 12040–12045 (2010).
Tamada, T. et al. Substrate recognition and selectivity of plant glycerol-3-phosphate acyltransferases (GPATs) from Cucurbita moscata and Spinacea oleracea. Acta Crystallogr. D 60, 13–21 (2004).
Oakes, J. et al. Expression of fungal diacylglycerol acyltransferase2 Genes to increase kernel oil in maize. Plant Physiol. 155, 1146–1157 (2011).
Bouvier-Navé, P., Benveniste, P., Oelkers, P., Sturley, S.L. & Schaller, H. Expression in yeast and tobacco of plant cDNAs encoding acyl CoA: diacylglycerol acyltransferase. FEBS J. 267, 85–96 (2011).
Lung, S.C. & Weselake, R.J. Diacylglycerol acyltransferase: a key mediator of plant triacylglycerol synthesis. Lipids 41, 1073–1088 (2005).
Xu, J.Y. et al. Cloning and characterization of an acyl–CoA–dependent diacylglycerol acyltransferase 1 (DGAT1) gene from Tropaeolum majus, and a study of the functional motifs of the DGAT protein using site–directed mutagenesis to modify enzyme activity and oil content. Plant Biotechnol. J. 6, 799–818 (2008).
Nickel, W., Brugger, B. & Wieland, F.T. Protein and lipid sorting between the endoplasmic reticulum and the Golgi complex. Semin. Cell Dev. Biol. 9, 493–501 (1998).
Mikkilineni, V. & Rocheford, T.R. Sequence variation and genomic organization of fatty acid desaturase–2 (fad2) and fatty acid desaturase–6 (fad6) cDNAs in maize. Theor. Appl. Genet. 106, 1326–1332 (2003).
Wassom, J.J., Mikkelineni, V., Bohn, M.O. & Rocheford, T.R. QTL for fatty acid composition of maize kernel oil in Illinois High Oil x B73 backcross–derived lines. Crop Sci. 48, 69–78 (2007).
Okuley, J. et al. Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 6, 147–158 (1994).
Dyer, J.M. & Mullen, R.T. Immunocytological localization of two plant fatty acid desaturases in the endoplasmic reticulum. FEBS Lett. 494, 44–47 (2001).
Li, Q. et al. Genome-wide association study identifies three independent polymorphisms for α-tocopherol content in maize kernels. PLoS ONE 7, e36807 (2012).
Edgar, R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Hall, T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Bradbury, P.J. et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635 (2007).
Yan, J.B. et al. High-throughput SNP genotyping with the GoldenGate assay in maize. Mol. Breed. 25, 441–451 (2010).
Lincoln, S.E., Daly, M.J. & Lander, E.S. Construction genetic maps with MAPMAKER/EXP 3.0. Whitehead Institute Technical Report, 3rd edn (Whitehead Institute, 1992).
Zeng, Z.B. Precision mapping of quantitative trait loci. Genetics 136, 1457–1468 (1994).
Wang, S., Basten, C.J. & Zeng, Z. Windows QTL Cartographer 2.5. (North Carolina State University, Raleigh, North Carolina, USA, 2005).
Ihaka, R. & Gentleman, R.R. A language for data analysis and graphics. J. Comput. Graph. Statist. 5, 299–314 (1996).
Smoot, M.E., Ono, K., Ruscheinski, J., Wang, P.L. & Ideker, T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27, 431–432 (2011).
Acknowledgements
We appreciated the helpful comments on the manuscript from A. Rafalski, J. Dudley, T. Setter and B. Shen. This research was supported by the National Hi-Tech Research and Development Program of China (2012AA10A307), the National Natural Science Foundation of China (31101156, 31123009), the National Basic Research Program of China (2009CB118404), the Ministry of Agriculture of China (2011ZX08009-001) and the National Gene Bank Project of China.
Author information
Authors and Affiliations
Contributions
J.Y., X.Y., J. Li and G.W. designed and supervised this study. H.L., W.W., Y.H., Y. Chai, P.Z. and X.H. performed the experiments. H.L., X.Y., W.W., Z.P., J.F., T.G., N.Y., Y.L., J. Liu, Y. Cheng and J.Y. analyzed data. J.W. and J.Z. contributed new regents. J.Y., H.L. and M.L.W. prepared the manuscript, and all the authors critically read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13, Supplementary Tables 1–11 (PDF 5365 kb)
Rights and permissions
About this article
Cite this article
Li, H., Peng, Z., Yang, X. et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat Genet 45, 43–50 (2013). https://doi.org/10.1038/ng.2484
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2484
This article is cited by
-
Reveal the kernel dehydration mechanisms in maize based on proteomic and metabolomic analysis
BMC Plant Biology (2024)
-
Common and specific genetic basis of metabolite-mediated drought responses in rice
Stress Biology (2024)
-
A genome-wide association study for resistance to Fusarium wilt (Fusarium oxysporum f. sp. vasinfectum) race 4 in diploid cotton (Gossypium arboreum) and resistance transfer to tetraploid Gossypium hirsutum
Molecular Genetics and Genomics (2024)
-
Identification of fusarium head blight resistance markers in a genome-wide association study of CIMMYT spring synthetic hexaploid derived wheat lines
BMC Plant Biology (2023)
-
Regulation of seed oil accumulation by lncRNAs in Brassica napus
Biotechnology for Biofuels and Bioproducts (2023)