Abstract
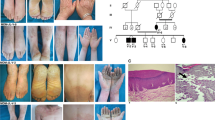
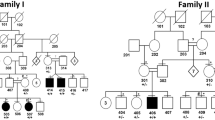
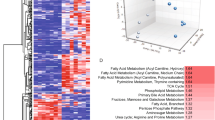
Palmoplantar keratodermas (PPKs) are a group of disorders that are diagnostically and therapeutically problematic in dermatogenetics1,2,3. Punctate PPKs are characterized by circumscribed hyperkeratotic lesions on the palms and soles with considerable heterogeneity. In 18 families with autosomal dominant punctate PPK, we report heterozygous loss-of-function mutations in AAGAB, encoding α- and γ-adaptin–binding protein p34, located at a previously linked locus at 15q22. α- and γ-adaptin–binding protein p34, a cytosolic protein with a Rab-like GTPase domain, was shown to bind both clathrin adaptor protein complexes, indicating a role in membrane trafficking. Ultrastructurally, lesional epidermis showed abnormalities in intracellular vesicle biology. Immunohistochemistry showed hyperproliferation within the punctate lesions. Knockdown of AAGAB in keratinocytes led to increased cell division, which was linked to greatly elevated epidermal growth factor receptor (EGFR) protein expression and tyrosine phosphorylation. We hypothesize that p34 deficiency may impair endocytic recycling of growth factor receptors such as EGFR, leading to increased signaling and cellular proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Itin, P.H. & Fistarol, S.K. Palmoplantar keratodermas. Clin. Dermatol. 23, 15–22 (2005).
Stevens, H.P. et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. Literature survey and proposed updated classification of the keratodermas. Arch. Dermatol. 132, 640–651 (1996).
Kelsell, D.P. & Stevens, H.P. The palmoplantar keratodermas: much more than palms and soles. Mol. Med. Today 5, 107–113 (1999).
Emmert, S. et al. 47 patients in 14 families with the rare genodermatosis keratosis punctata palmoplantaris Buschke-Fischer-Brauer. Eur. J. Dermatol. 13, 16–20 (2003).
Martinez-Mir, A. et al. Identification of a locus for type I punctate palmoplantar keratoderma on chromosome 15q22-q24. J. Med. Genet. 40, 872–878 (2003).
Gao, M. et al. Refined localization of a punctate palmoplantar keratoderma gene to a 5.06-cM region at 15q22.2–15q22.31. Br. J. Dermatol. 152, 874–878 (2005).
Jung, E.G. Acrokeratoelastoidosis. Humangenetik 17, 357–358 (1973).
Zhang, X.J. et al. Identification of a locus for punctate palmoplantar keratodermas at chromosome 8q24.13–8q24.21. J. Invest. Dermatol. 122, 1121–1125 (2004).
El Amri, I. et al. Clinical and genetic characteristics of Buschke-Fischer-Brauer's disease in a Tunisian family. Ann. Dermatol. Venereol. 137, 269–275 (2010).
Page, L.J., Sowerby, P.J., Lui, W.W. & Robinson, M.S. γ-synergin: an EH domain–containing protein that interacts with γ-adaptin. J. Cell Biol. 146, 993–1004 (1999).
Boukamp, P. et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106, 761–771 (1988).
Robinson, M.S. & Bonifacino, J.S. Adaptor-related proteins. Curr. Opin. Cell Biol. 13, 444–453 (2001).
Robinson, M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167–174 (2004).
Horgan, C.P. & McCaffrey, M.W. Rab GTPases and microtubule motors. Biochem. Soc. Trans. 39, 1202–1206 (2011).
Ceresa, B.P. Regulation of EGFR endocytic trafficking by rab proteins. Histol. Histopathol. 21, 987–993 (2006).
Rappoport, J.Z. & Simon, S.M. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J. Cell Sci. 122, 1301–1305 (2009).
Downward, J., Parker, P. & Waterfield, M.D. Autophosphorylation sites on the epidermal growth factor receptor. Nature 311, 483–485 (1984).
Sousa, L.P. et al. Suppression of EGFR endocytosis by dynamin depletion reveals that EGFR signaling occurs primarily at the plasma membrane. Proc. Natl. Acad. Sci. USA 109, 4419–4424 (2012).
Goh, L.K., Huang, F., Kim, W., Gygi, S. & Sorkin, A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J. Cell Biol. 189, 871–883 (2010).
Sprecher, E. et al. A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neurocutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am. J. Hum. Genet. 77, 242–251 (2005).
Gissen, P. et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis–renal dysfunction–cholestasis (ARC) syndrome. Nat. Genet. 36, 400–404 (2004).
Montpetit, A. et al. Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet. 4, e1000296 (2008).
Van Gele, M., Dynoodt, P. & Lambert, J. Griscelli syndrome: a model system to study vesicular trafficking. Pigment Cell Melanoma Res. 22, 268–282 (2009).
Tarpey, P.S. et al. Mutations in the gene encoding the Sigma 2 subunit of the adaptor protein 1 complex, AP1S2, cause X-linked mental retardation. Am. J. Hum. Genet. 79, 1119–1124 (2006).
Bennion, S.D. & Patterson, J.W. Keratosis punctata palmaris et plantaris and adenocarcinoma of the colon. A possible familial association of punctate keratoderma and gastrointestinal malignancy. J. Am. Acad. Dermatol. 10, 587–591 (1984).
Armstrong, D.K. et al. Haploinsufficiency of desmoplakin causes a striate subtype of palmoplantar keratoderma. Hum. Mol. Genet. 8, 143–148 (1999).
Wan, H. et al. Striate palmoplantar keratoderma arising from desmoplakin and desmoglein 1 mutations is associated with contrasting perturbations of desmosomes and the keratin filament network. Br. J. Dermatol. 150, 878–891 (2004).
McLean, W.H. Genetic disorders of palm skin and nail. J. Anat. 202, 133–141 (2003).
Cottingham, R.W. Jr., Idury, R.M. & Schaffer, A.A. Faster sequential genetic linkage computations. Am. J. Hum. Genet. 53, 252–263 (1993).
Schäffer, A.A., Gupta, S.K., Shriram, K. & Cottingham, R.W. Jr. Avoiding recomputation in linkage analysis. Hum. Hered. 44, 225–237 (1994).
Hirst, J., Miller, S.E., Taylor, M.J., von Mollard, G.F. & Robinson, M.S. EpsinR is an adaptor for the SNARE protein Vti1b. Mol. Biol. Cell 15, 5593–5602 (2004).
Acknowledgements
The authors dedicate this paper to their erstwhile colleague, the late dermatologist and cell biologist Susan M. Morley, who treated some of the individuals studied here. We thank M. Robinson and C. Watts for insightful discussions, I. Nathke and I. Newton for their help with protein blot quantification and Tayside Tissue Bank, Dundee for providing skin samples. Specialist Sequencing and Bioinformatics Services were provided by The Eastern Sequence and Informatics Hub (EASIH) at the University of Cambridge, which is supported by the National Institute for Health Research and the Cambridge Biomedical Research Centre. This work was supported by a Wellcome Trust Programme Grant (092530/Z/10/Z) to W.H.I.M., A.D.I. and G.J.B., a Wellcome Trust Strategic Award (098439/Z/12/Z) to W.H.I.M., G.J.B. and J.A.M., a project grant from the Pachyonychia Congenita Project to F.J.D.S. and a strategic positioning fund for Genetic Orphan Diseases from A*STAR. O.M. was funded by an A*STAR Research Attachment Program (ARAP), and B.R. is a fellow of the Branco Weiss Foundation.
Author information
Authors and Affiliations
Contributions
W.H.I.M. designed the study. M.Z., H.H., T.N., A.D.I., B.M., H.S., M.A., M. Suehiro, I.K., L.B., M.D., A. Saad, M.G., O.M. and C.S.M. diagnosed subjects and collected clinical samples and phenotype data. E.P., O.M., N.J.W., M. Shboul and S.T. conducted genotyping, mapping and sequencing. J.H. and E.P. performed protein functional studies. J.H. generated the polyclonal antibodies to p34. C.C. and G.J.B. carried out next-generation sequencing bioinformatics. A.T.E. performed the dermatopathology analysis. P.J.D.-H. and J.A.M. performed ultrastructural analysis. S.J.B., O.M. and A. Sandilands provided tissue samples. C.S.M.G. and A. Sandilands performed the tissue expression analysis. D.R.G. performed statistical genetics. W.H.I.M., E.P., J.H., B.R., J.A.M., C.S.M. and F.J.D.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–5 (PDF 7296 kb)
Rights and permissions
About this article
Cite this article
Pohler, E., Mamai, O., Hirst, J. et al. Haploinsufficiency for AAGAB causes clinically heterogeneous forms of punctate palmoplantar keratoderma. Nat Genet 44, 1272–1276 (2012). https://doi.org/10.1038/ng.2444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2444
This article is cited by
-
Is punctate palmoplantar keratoderma type 1 associated with malignancy? A systematic review of the literature
Orphanet Journal of Rare Diseases (2023)
-
Adaptor protein complex interaction map in Arabidopsis identifies P34 as a common stability regulator
Nature Plants (2023)
-
On-treatment biomarkers can improve prediction of response to neoadjuvant chemotherapy in breast cancer
Breast Cancer Research (2019)
-
Clathrin Adaptor Complex-interacting Protein Irc6 Functions through the Conserved C-Terminal Domain
Scientific Reports (2019)
-
Multiple Self-Healing Palmoplantar Carcinoma: A Familial Predisposition to Skin Cancer with Primary Palmoplantar and Conjunctival Lesions
Journal of Investigative Dermatology (2015)