Abstract
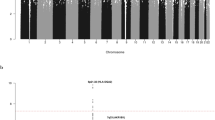
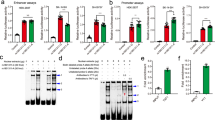
Neuroblastoma is a cancer of the sympathetic nervous system that accounts for approximately 10% of all pediatric oncology deaths1. Here, we report a genome-wide association study of 2,817 neuroblastoma cases and 7,473 controls. We identified two new associations at 6q16, the first within HACE1 (rs4336470; combined P = 2.7 × 10−11; odds ratio 1.26, 95% confidence interval (CI) 1.18–1.35) and the second within LIN28B (rs17065417; combined P = 1.2 × 10−8; odds ratio 1.38, 95% CI 1.23–1.54). Expression of LIN28B and let-7 miRNA correlated with rs17065417 genotype in neuroblastoma cell lines, and we observed significant growth inhibition upon depletion of LIN28B, specifically in neuroblastoma cells that were homozygous for the risk allele. Low HACE1 and high LIN28B expression in diagnostic primary neuroblastomas were associated with worse overall survival (P = 0.008 and 0.014, respectively). Taken together, these data show that common variants in HACE1 and LIN28B influence neuroblastoma susceptibility and indicate that both genes likely have a role in disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 362, 2202–2211 (2010).
Yu, A.L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 (2010).
Mossé, Y.P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–935 (2008).
Janoueix-Lerosey, I. et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455, 967–970 (2008).
Trochet, D. et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Am. J. Hum. Genet. 74, 761–764 (2004).
Mosse, Y.P. et al. Germline PHOX2B mutation in hereditary neuroblastoma. Am. J. Hum. Genet. 75, 727–730 (2004).
Maris, J.M. et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N. Engl. J. Med. 358, 2585–2593 (2008).
Capasso, M. et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat. Genet. 41, 718–723 (2009).
Wang, K. et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature 469, 216–220 (2011).
Nguyen, B. et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility loci. PLoS Genet. 7, e1002026 (2011).
Diskin, S.J. et al. Copy number variation at 1q21.1 associated with neuroblastoma. Nature 459, 987–991 (2009).
Latorre, V. et al. Replication of neuroblastoma SNP association at the BARD1 locus in African-Americans. Cancer Epidemiol. Biomarkers Prev. 21, 658–663 (2012).
Henderson, T.O. et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children's Oncology Group study. J. Clin. Oncol. 29, 76–82 (2011).
Willer, C.J., Li, Y. & Abecasis, G.R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Anglesio, M.S. et al. Differential expression of a novel ankyrin containing E3 ubiquitin-protein ligase, Hace1, in sporadic Wilms' tumor versus normal kidney. Hum. Mol. Genet. 13, 2061–2074 (2004).
Hibi, K. et al. Aberrant methylation of the HACE1 gene is frequently detected in advanced colorectal cancer. Anticancer Res. 28, 1581–1584 (2008).
Sakata, M. et al. Methylation of HACE1 in gastric carcinoma. Anticancer Res. 29, 2231–2233 (2009).
Zhang, L. et al. The E3 ligase HACE1 is a critical chromosome 6q21 tumor suppressor involved in multiple cancers. Nat. Med. 13, 1060–1069 (2007).
Zhao, J., Zhang, Z., Vucetic, Z., Soprano, K.J. & Soprano, D.R. HACE1: a novel repressor of RAR transcriptional activity. J. Cell. Biochem. 107, 482–493 (2009).
Slade, I. et al. Constitutional translocation breakpoint mapping by genome-wide paired-end sequencing identifies HACE1 as a putative Wilms tumour susceptibility gene. J. Med. Genet. 47, 342–347 (2010).
Piskounova, E. et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 147, 1066–1079 (2011).
Iliopoulos, D., Hirsch, H.A. & Struhl, K. An epigenetic switch involving NF-κB, Lin28, Let-7 microRNA, and IL6 links inflammation to cell transformation. Cell 139, 693–706 (2009).
West, J.A. et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 460, 909–913 (2009).
Viswanathan, S.R. et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat. Genet. 41, 843–848 (2009).
Martinez, N.J. & Gregory, R.I. MicroRNA gene regulatory pathways in the establishment and maintenance of ESC identity. Cell Stem Cell 7, 31–35 (2010).
Melton, C., Judson, R.L. & Blelloch, R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463, 621–626 (2010).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Neale, G. et al. Molecular characterization of the pediatric preclinical testing panel. Clin. Cancer Res. 14, 4572–4583 (2008).
Zhu, H. et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94 (2011).
Yuan, J., Nguyen, C.K., Liu, X., Kanellopoulou, C. & Muljo, S.A. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335, 1195–1200 (2012).
Widén, E. et al. Distinct variants at LIN28B influence growth in height from birth to adulthood. Am. J. Hum. Genet. 86, 773–782 (2010).
Ong, K.K. et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat. Genet. 41, 729–733 (2009).
Sulem, P. et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat. Genet. 41, 734–738 (2009).
Permuth-Wey, J. et al. LIN28B polymorphisms influence susceptibility to epithelial ovarian cancer. Cancer Res. 71, 3896–3903 (2011).
Bosse, K.R. et al. Common variation at BARD1 results in the expression of an oncogenic isoform that influences neuroblastoma susceptibility and oncogenicity. Cancer Res. 72, 2068–2078 (2012).
Pruim, R.J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010).
Molenaar, J.J. et al. Copy number defects of G1-cell cycle genes in neuroblastoma are frequent and correlate with high expression of E2F target genes and a poor prognosis. Genes Chromosomes Cancer 51, 10–19 (2012).
Brodeur, G.M. et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 11, 1466–1477 (1993).
Shimada, H. et al. The International Neuroblastoma Pathology Classification (Shimada) System. Cancer 86, 364–372 (1999).
Mathew, P. et al. Detection of MYCN gene amplification in neuroblastoma by fluorescence in situ hybridization: a Pediatric Oncology Group study. Neoplasia 3, 105–109 (2001).
Look, A.T. et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J. Clin. Oncol. 9, 581–591 (1991).
Gunderson, K.L., Steemers, F.J., Lee, G., Mendoza, L.G. & Chee, M.S. A genome-wide scalable SNP genotyping assay using microarray technology. Nat. Genet. 37, 549–554 (2005).
Steemers, F.J. et al. Whole-genome genotyping with the single-base extension assay. Nat. Methods 3, 31–33 (2006).
Howie, B.N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Howie, B., Marchini, J. & Stephens, M. Genotype imputation with thousands of genomes. G3 (Bethesda) 1, 457–470 (2011).
Marchini, J., Howie, B., Myers, S., McVean, G. & Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 39, 906–913 (2007).
Cole, K.A. et al. A functional screen identifies miR-34a as a candidate neuroblastoma tumor suppressor gene. Mol. Cancer Res. 6, 735–742 (2008).
Kaplan, E.L. & Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assn. 53, 457–481 (1958).
Acknowledgements
We thank P. Sleiman for useful discussions regarding meta-analysis using METAL. This work was supported in part by US National Institutes of Health (NIH) grants R01-CA124709 (J.M.M.), K99-CA151869 (S.J.D.), P30-HD026979 (M. Devoto) and K08-CA136979 (K.A.C.); the Giulio D'Angio Endowed Chair (J.M.M.); the Alex's Lemonade Stand Foundation (J.M.M.); Andrew's Army Foundation (J.M.M.); the PressOn Foundation (J.M.M.); the Abramson Family Cancer Research Institute (J.M.M.); Fondazione Italiana per la Lotta al Neuroblastoma and Associazione Italiana per la Ricerca sul Cancro (M.C.); and the Center for Applied Genomics at the Children's Hospital of Philadelphia Research Institute (H.H.).
Author information
Authors and Affiliations
Contributions
S.J.D. and J.M.M. designed the experiment and drafted the manuscript. S.J.D. analyzed SNP data, performed SNP association studies, and analyzed mRNA and miRNA expression data. M.C. and A.I. replicated SNP associations in the Italian cohort. V.L. and M.D. replicated SNP associations in the African-American cohort. E.L.C. and H.L. confirmed LIN28B protein expression by protein blot. R.W.S. and C.W. performed experiments with siRNA-mediated knockdown of LIN28B. E.F.A. generated miRNA expression array data, including low-level summary values. K.A.C. performed RT-PCR in primary tumors. M. Diamond and C.H. organized samples and genotyped cases. J.J. participated in expression array analyses. H.H. generated and provided all control data for the GWAS. M. Devoto and H.H. contributed to overall study design. All authors commented on or contributed to the current manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–10 and Supplementary Figures 1–12 (PDF 1681 kb)
Rights and permissions
About this article
Cite this article
Diskin, S., Capasso, M., Schnepp, R. et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet 44, 1126–1130 (2012). https://doi.org/10.1038/ng.2387
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2387
This article is cited by
-
Advancing therapy for neuroblastoma
Nature Reviews Clinical Oncology (2022)
-
MYCN mediates TFRC-dependent ferroptosis and reveals vulnerabilities in neuroblastoma
Cell Death & Disease (2021)
-
MYC-associated protein X binding with the variant rs72780850 in RNA helicase DEAD box 1 for susceptibility to neuroblastoma
Science China Life Sciences (2021)
-
Mouse models of high-risk neuroblastoma
Cancer and Metastasis Reviews (2020)
-
Genetic predisposition and chromosome instability in neuroblastoma
Cancer and Metastasis Reviews (2020)