Abstract
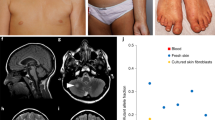
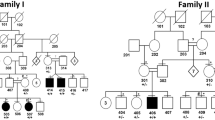
Nevus sebaceous is a common congenital cutaneous malformation. Affected individuals may develop benign and malignant secondary tumors in the nevi during life. Schimmelpenning syndrome is characterized by the association of nevus sebaceous with extracutaneous abnormalities. We report that of 65 sebaceous nevi studied, 62 (95%) had mutations in the HRAS gene and 3 (5%) had mutations in the KRAS gene. The HRAS c.37G>C mutation, which results in a p.Gly13Arg substitution, was present in 91% of lesions. Nonlesional tissues from 18 individuals had a wild-type sequence, confirming genetic mosaicism. The HRAS c.37G>C mutation was also found in 8 of 8 associated secondary tumors. Mosaicism for HRAS c.37G>C and KRAS c.35G>A mutations was found in two individuals with Schimmelpenning syndrome. Functional analysis of HRAS c.37G>C mutant cells showed constitutive activation of the MAPK and PI3K-Akt signaling pathways. Our results indicate that nevus sebaceous and Schimmelpenning syndrome are caused by postzygotic HRAS and KRAS mutations. These mutations may predispose individuals to the development of secondary tumors in nevus sebaceous.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sugarman, J.L. Epidermal nevus syndromes. Semin. Cutan. Med. Surg. 26, 221–230 (2007).
Rogers, M. Epidermal nevi and the epidermal nevus syndromes: a review of 233 cases. Pediatr. Dermatol. 9, 342–344 (1992).
Moody, M.N., Landau, J.M. & Goldberg, L.H. Nevus sebaceous revisited. Pediatr. Dermatol. 29, 15–23 (2012).
Izumi, M. et al. Ten cases of sebaceous carcinoma arising in nevus sebaceus. J. Dermatol. 35, 704–711 (2008).
Ball, E.A., Hussain, M. & Moss, A.L. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin. Exp. Dermatol. 30, 259–260 (2005).
Chou, C.Y., Chen, W.Y., Wang, K.H. & Chen, T.J. Carcinosarcoma derived from nevus sebaceus. J. Clin. Oncol. 29, e719–e721 (2011).
Schimmelpenning, G.W. Clinical contribution to symptomatology of phacomatosis [in German]. Fortschr. Geb. Rontgenstr. Nuklearmed. 87, 716–720 (1957).
Davies, D. & Rogers, M. Review of neurological manifestations in 196 patients with sebaceous naevi. Australas. J. Dermatol. 43, 20–23 (2002).
Happle, R. The group of epidermal nevus syndromes Part I. Well-defined phenotypes. J. Am. Acad. Dermatol. 63, 1–22 (2010).
Happle, R. Mosaicism in human skin. Understanding the patterns and mechanisms. Arch. Dermatol. 129, 1460–1470 (1993).
Hafner, C. et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc. Natl. Acad. Sci. USA 104, 13450–13454 (2007).
Hafner, C. et al. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J. Clin. Invest. 116, 2201–2207 (2006).
Zutt, M. et al. Schimmelpenning-Feuerstein-Mims syndrome with hypophosphatemic rickets. Dermatology 207, 72–76 (2003).
Rijntjes-Jacobs, E.G., Lopriore, E., Steggerda, S.J., Kant, S.G. & Walther, F.J. Discordance for Schimmelpenning-Feuerstein-Mims syndrome in monochorionic twins supports the concept of a postzygotic mutation. Am. J. Med. Genet. A 152A, 2816–2819 (2010).
Aoki, Y. et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat. Genet. 37, 1038–1040 (2005).
Gripp, K.W. & Lin, A.E. Costello syndrome: A Ras/mitogen activated protein kinase pathway syndrome (rasopathy) resulting from HRAS germline mutations. Genet. Med. 14, 285–292 (2012).
Hafner, C., Toll, A. & Real, F.X. HRAS mutation mosaicism causing urothelial cancer and epidermal nevus. N. Engl. J. Med. 365, 1940–1942 (2011).
Gripp, K.W., Stabley, D.L., Nicholson, L., Hoffman, J.D. & Sol-Church, K. Somatic mosaicism for an HRAS mutation causes Costello syndrome. Am. J. Med. Genet. A 140, 2163–2169 (2006).
Sol-Church, K. et al. Male-to-male transmission of Costello syndrome: G12S HRAS germline mutation inherited from a father with somatic mosaicism. Am. J. Med. Genet. A 149A, 315–321 (2009).
Tuveson, D.A. et al. Endogenous oncogenic K-rasG12D stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 (2004).
Bourdeaut, F. et al. Mosaicism for oncogenic G12D KRAS mutation associated with epidermal nevus, polycystic kidneys and rhabdomyosarcoma. J. Med. Genet. 47, 859–862 (2010).
Niihori, T. et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat. Genet. 38, 294–296 (2006).
Schubbert, S. et al. Germline KRAS mutations cause Noonan syndrome. Nat. Genet. 38, 331–336 (2006).
Schubbert, S. et al. Biochemical and functional characterization of germ line KRAS mutations. Mol. Cell. Biol. 27, 7765–7770 (2007).
Happle, R. Lethal genes surviving by mosaicism: a possible explanation for sporadic birth defects involving the skin. J. Am. Acad. Dermatol. 16, 899–906 (1987).
Hafner, C. et al. High frequency of FGFR3 mutations in adenoid seborrheic keratoses. J. Invest. Dermatol. 126, 2404–2407 (2006).
Hafner, C. et al. Multiple oncogenic mutations and clonal relationship in spatially distinct benign human epidermal tumors. Proc. Natl. Acad. Sci. USA 107, 20780–20785 (2010).
Lindhurst, M.J. et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N. Engl. J. Med. 365, 611–619 (2011).
Amary, M.F. et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat. Genet. 43, 1262–1265 (2011).
Pansuriya, T.C. et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat. Genet. 43, 1256–1261 (2011).
Tidyman, W.E. & Rauen, K.A. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev. 19, 230–236 (2009).
Kompier, L.C. et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS ONE 5, e13821 (2010).
Hurst, C.D., Zuiverloon, T.C., Hafner, C., Zwarthoff, E.C. & Knowles, M.A.A. SNaPshot assay for the rapid and simple detection of four common hotspot codon mutations in the PIK3CA gene. BMC Res. Notes 2, 66 (2009).
Acknowledgements
This work was supported by the research grant HA 5531/1-2 from the Deutsche Forschungsgemeinschaft to C.H. We thank all subjects who participated in this study and A. Groesser and H. Hafner for their valuable support.
Author information
Authors and Affiliations
Contributions
L.G. collected study material, performed microdissection, supervised functional analyses, analyzed and discussed the data and participated in writing the manuscript. E.H. performed microdissection, genetic analyses, cell culture, protein blots and assays and analyzed the data. A.R., C.R., E.L. and M.Z. contributed material and discussed the data. T.L. was involved in the functional characterization of the mutation and discussed the data. S.S., A.T. and M.L. analyzed and discussed the data. L.K. and W.S.-B. contributed to transfection experiments and analyzed the data. F.X.R. analyzed and discussed the data and participated in writing the manuscript. C.H. designed the study, collected study material, supervised all experimental work, analyzed and discussed the data, obtained financial support and wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2; Supplementary Figure 1 (PDF 192 kb)
Rights and permissions
About this article
Cite this article
Groesser, L., Herschberger, E., Ruetten, A. et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet 44, 783–787 (2012). https://doi.org/10.1038/ng.2316
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2316
This article is cited by
-
Expansion of the complex genotypic and phenotypic spectrum of FGFR2-associated neurocutaneous syndromes
Human Genetics (2024)
-
Signalling by senescent melanocytes hyperactivates hair growth
Nature (2023)
-
Naevus Sebaceous of Jadassohn
Indian Journal of Pediatrics (2021)
-
Review of Pediatric Head and Neck Neoplasms that Raise the Possibility of a Cancer Predisposition Syndrome
Head and Neck Pathology (2021)
-
Identification of KRAS mutation in a patient with linear nevus sebaceous syndrome: a case report
BMC Medical Genomics (2020)