Abstract
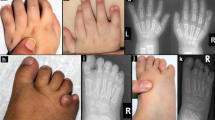
IMAGe syndrome (intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia congenita and genital anomalies) is an undergrowth developmental disorder with life-threatening consequences1. An identity-by-descent analysis in a family with IMAGe syndrome2 identified a 17.2-Mb locus on chromosome 11p15 that segregated in the affected family members. Targeted exon array capture of the disease locus, followed by high-throughput genomic sequencing and validation by dideoxy sequencing, identified missense mutations in the imprinted gene CDKN1C (also known as P57KIP2) in two familial and four unrelated patients. A familial analysis showed an imprinted mode of inheritance in which only maternal transmission of the mutation resulted in IMAGe syndrome. CDKN1C inhibits cell-cycle progression3, and we found that targeted expression of IMAGe-associated CDKN1C mutations in Drosophila caused severe eye growth defects compared to wild-type CDKN1C, suggesting a gain-of-function mechanism. All IMAGe-associated mutations clustered in the PCNA-binding domain of CDKN1C and resulted in loss of PCNA binding, distinguishing them from the mutations of CDKN1C that cause Beckwith-Wiedemann syndrome, an overgrowth syndrome4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vilain, E. et al. IMAGe, a new clinical association of intrauterine growth retardation, metaphyseal dysplasia, adrenal hypoplasia congenita, and genital anomalies. J. Clin. Endocrinol. Metab. 84, 4335–4340 (1999).
Bergadá, I. et al. Familial occurrence of the IMAGe association: additional clinical variants and a proposed mode of inheritance. J. Clin. Endocrinol. Metab. 90, 3186–3190 (2005).
Lee, M.H., Reynisdottir, I. & Massagué, J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 9, 639–649 (1995).
Romanelli, V. et al. CDKN1C (p57Kip2) analysis in Beckwith-Wiedemann syndrome (BWS) patients: genotype-phenotype correlations, novel mutations, and polymorphisms. Am. J. Med. Genet. A. 152A, 1390–1397 (2010).
Hutz, J.E. et al. IMAGe association and congenital adrenal hypoplasia: no disease-causing mutations found in the ACD gene. Mol. Genet. Metab. 88, 66–70 (2006).
Ko, J.M., Lee, J.H., Kim, G.H., Kim, A.R. & Yoo, H.W. A case of a Korean newborn with IMAGe association presenting with hyperpigmented skin at birth. Eur. J. Pediatr. 166, 879–880 (2007).
Lienhardt, A., Mas, J.C., Kalifa, G., Chaussain, J.L. & Tauber, M. IMAGe association: additional clinical features and evidence for recessive autosomal inheritance. Horm. Res. 57 (suppl. 2), 71–78 (2002).
Pedreira, C.C., Savarirayan, R. & Zacharin, M.R. IMAGe syndrome: a complex disorder affecting growth, adrenal and gonadal function, and skeletal development. J. Pediatr. 144, 274–277 (2004).
Tan, T.Y. et al. Two sisters with IMAGe syndrome: cytomegalic adrenal histopathology, support for autosomal recessive inheritance and literature review. Am. J. Med. Genet. A. 140, 1778–1784 (2006).
Amano, N. et al. Radiological evolution in IMAGe association: a case report. Am. J. Med. Genet. A. 146A, 2130–2133 (2008).
Balasubramanian, M., Sprigg, A. & Johnson, D.S. IMAGe syndrome: case report with a previously unreported feature and review of published literature. Am. J. Med. Genet. A. 152A, 3138–3142 (2010).
Watanabe, H. et al. Suppression of cell transformation by the cyclin-dependent kinase inhibitor p57KIP2 requires binding to proliferating cell nuclear antigen. Proc. Natl. Acad. Sci. USA 95, 1392–1397 (1998).
Goujon, M. et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 38, W695–W699 (2010).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Diaz-Meyer, N. et al. Silencing of CDKN1C (p57KIP2) is associated with hypomethylation at KvDMR1 in Beckwith-Wiedemann syndrome. J. Med. Genet. 40, 797–801 (2003).
Shin, J.Y., Fitzpatrick, G.V. & Higgins, M.J. Two distinct mechanisms of silencing by the KvDMR1 imprinting control region. EMBO J. 27, 168–178 (2008).
Brand, A.H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Wiedemann, H.R. The EMG-syndrome: exomphalos, macroglossia, gigantism and disturbed carbohydrate metabolism. Z. Kinderheilkd. 106, 171–185 (1969).
Beckwith, J.B. Macroglossia, omphalocele, adrenal cytomegaly, gigantism, and hyperplastic visceromegaly. Birth Defects Orig. Art. Ser. 2, 188–196 (1969).
Zhang, P. et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 387, 151–158 (1997).
Hatada, I. et al. An imprinted gene p57KIP2 is mutated in Beckwith-Wiedemann syndrome. Nat. Genet. 14, 171–173 (1996).
Bourcigaux, N. et al. High expression of cyclin E and G1 CDK and loss of function of p57KIP2 are involved in proliferation of malignant sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 85, 322–330 (2000).
Havens, C.G. & Walter, J.C. Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 25, 1568–1582 (2011).
Kirchmaier, A.L. Ub-family modifications at the replication fork: regulating PCNA-interacting components. FEBS Lett. 585, 2920–2928 (2011).
Ye, Y. & Rape, M. Building ubiquitin chains: E2 enzymes at work. Nat. Rev. Mol. Cell Biol. 10, 755–764 (2009).
Mukhopadhyay, D. & Riezman, H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315, 201–205 (2007).
Li, W. & Ye, Y. Polyubiquitin chains: functions, structures, and mechanisms. Cell. Mol. Life Sci. 65, 2397–2406 (2008).
Thrower, J.S., Hoffman, L., Rechsteiner, M. & Pickart, C.M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 19, 94–102 (2000).
Lee, H., Jen, J.C., Cha, Y.H., Nelson, S.F. & Baloh, R.W. Phenotypic and genetic analysis of a large family with migraine-associated vertigo. Headache 48, 1460–1467 (2008).
Lee, H. et al. Improving the efficiency of genomic loci capture using oligonucleotide arrays for high throughput resequencing. BMC Genomics 10, 646 (2009).
Homer, N., Merriman, B. & Nelson, S.F. BFAST: an alignment tool for large scale genome resequencing. PLoS ONE 4, e7767 (2009).
Homer, N., Merriman, B. & Nelson, S.F. Local alignment of two-base encoded DNA sequence. BMC Bioinformatics 10, 175 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Clark, M.J. et al. U87MG decoded: the genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS Genet. 6, e1000832 (2010).
O'Connor, B.D., Merriman, B. & Nelson, S.F. SeqWare Query Engine: storing and searching sequence data in the cloud. BMC Bioinformatics 11 (suppl. 12), S2 (2010).
Matsuoka, S. et al. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 9, 650–662 (1995).
Mattera, R., Tsai, Y.C., Weissman, A.M. & Bonifacino, J.S. The Rab5 guanine nucleotide exchange factor Rabex-5 binds ubiquitin (Ub) and functions as a Ub ligase through an atypical Ub-interacting motif and a zinc finger domain. J. Biol. Chem. 281, 6874–6883 (2006).
Acknowledgements
This work was funded by the Doris Duke Charitable Foundation and National Institute of Child Health and Human Development RO1HD068138. V.A.A. was supported by the US National Institutes of Health (NIH) 1 F31HD068136 training grant. The human embryonic and fetal material was provided by the Joint Medical Research Council (grant G0700089) and Wellcome Trust (grant GR082557) Human Developmental Biology Resource (www.hdbr.org). J.C.A. was supported by a Wellcome Trust Senior Research Fellowship in Clinical Science (079666). We thank R. Matera (NIH) for kindly providing the pCI-neo-(HA)3-human ubiquitin construct. We thank E.R.B. McCabe for initial crucial support of the adrenal research for E.V. and for providing one of the original IMAGe patient's DNA samples. We thank M. Le Merrer and C. Lecointre, who participated in the initial clinical description of IMAGe.
Author information
Authors and Affiliations
Contributions
V.A.A. designed and performed the experiments, analyzed data and wrote the paper. E.V. designed the project, supervised the overall experiments and wrote the paper with V.A.A. H.L. and S.F.N. contributed to design and analysis of the linkage and sequencing data. E.C. Délot contributed to the design of cell-cycle analysis experiments and editing of the manuscript. A.F., E.C. Dell'Angelica and I.A.R.-F. contributed to the nuclear localization experiments and design of the PCNA and ubiquitin assays. D.B. and I.B. clinically assessed and extracted DNA from family A. R.P., B.F.-d.-S. and J.C.A. performed immunofluorescence and RT-PCR experiments. A.B. and J.A.M.-A. performed, analyzed and contributed to the reporting of the Drosophila experiments. All authors discussed the results and implications of the work and commented on the manuscript at various stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Table 1. (PDF 1993 kb)
Rights and permissions
About this article
Cite this article
Arboleda, V., Lee, H., Parnaik, R. et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat Genet 44, 788–792 (2012). https://doi.org/10.1038/ng.2275
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2275
This article is cited by
-
Development and function of the fetal adrenal
Reviews in Endocrine and Metabolic Disorders (2023)
-
Rare forms of genetic paediatric adrenal insufficiency: Excluding congenital adrenal hyperplasia
Reviews in Endocrine and Metabolic Disorders (2023)
-
Genetic aetiology of primary adrenal insufficiency in Chinese children
BMC Medical Genomics (2021)
-
Contribution of gene mutations to Silver-Russell syndrome phenotype: multigene sequencing analysis in 92 etiology-unknown patients
Clinical Epigenetics (2020)
-
Novel mutation points to a hot spot in CDKN1C causing Silver–Russell syndrome
Clinical Epigenetics (2020)