Abstract
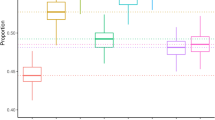
Schizophrenia is a complex disorder caused by both genetic and environmental factors. Using 9,087 affected individuals, 12,171 controls and 915,354 imputed SNPs from the Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium (PGC-SCZ), we estimate that 23% (s.e. = 1%) of variation in liability to schizophrenia is captured by SNPs. We show that a substantial proportion of this variation must be the result of common causal variants, that the variance explained by each chromosome is linearly related to its length (r = 0.89, P = 2.6 × 10−8), that the genetic basis of schizophrenia is the same in males and females, and that a disproportionate proportion of variation is attributable to a set of 2,725 genes expressed in the central nervous system (CNS; P = 7.6 × 10−8). These results are consistent with a polygenic genetic architecture and imply more individual SNP associations will be detected for this disease as sample size increases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
15 May 2012
In the version of this article initially published, the citation for the CNS+ gene set was incorrectly given as reference 17. The correct reference (Raychadhuri et al.) has been added as reference 28 in the HTML and PDF versions of the article.
References
Sullivan, P.F., Kendler, K.S. & Neale, M.C. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 60, 1187–1192 (2003).
Cardno, A.G. & Gottesman, I.I. Twin studies of schizophrenia: from bow-and-arrow concordances to Star Wars Mx and functional genomics. Am. J. Med. Genet. 97, 12–17 (2000).
Lichtenstein, P. et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373, 234–239 (2009).
McClellan, J.M., Susser, E. & King, M.C. Schizophrenia: a common disease caused by multiple rare alleles. Br. J. Psychiatry 190, 194–199 (2007).
Craddock, N., O'Donovan, M.C. & Owen, M.J. Phenotypic and genetic complexity of psychosis. Invited commentary on...Schizophrenia: a common disease caused by multiple rare alleles. Br. J. Psychiatry 190, 200–203 (2007).
McClellan, J. & King, M.C. Genetic heterogeneity in human disease. Cell 141, 210–217 (2010).
McClellan, J. & King, M.C. Genomic analysis of mental illness: a changing landscape. JAMA 303, 2523–2524 (2010).
Purcell, S.M. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009).
Shi, J. et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460, 753–757 (2009).
Moskvina, V. et al. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol. Psychiatry 14, 252–260 (2009).
Visscher, P.M., Goddard, M.E., Derks, E.M. & Wray, N.R. Evidence-based psychiatric genetics, AKA the false dichotomy between common and rare variant hypotheses. Mol. Psychiatry published online (14 June 2011), doi:10.1038/mp.2011.65.
Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42, 565–569 (2010).
Lee, S.H., Wray, N.R., Goddard, M.E. & Visscher, P.M. Estimating missing heritability for disease from genome-wide association studies. Am. J. Hum. Genet. 88, 294–305 (2011).
Ripke, S. et al. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 43, 969–976 (2011).
Yang, J. et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 43, 519–525 (2011).
Abel, K.M., Drake, R. & Goldstein, J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry 22, 417–428 (2010).
Lango Allen, H. et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838 (2010).
Cirulli, E.T. & Goldstein, D.B. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat. Rev. Genet. 11, 415–425 (2010).
Wray, N.R. Allele frequencies and the r2 measure of linkage disequilibrium: impact on design and interpretation of association studies. Twin Res. Hum. Genet. 8, 87–94 (2005).
Wray, N.R., Purcell, S.M. & Visscher, P.M. Synthetic associations created by rare variants do not explain most GWAS results. PLoS Biol. 9, e1000579 (2011).
Kang, H.M. et al. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 42, 348–354 (2010).
Yang, J., Wray, N.R. & Visscher, P.M. Comparing apples and oranges: equating the power of case-control and quantitative trait association studies. Genet. Epidemiol. 34, 254–257 (2010).
Powell, J.E., Visscher, P.M. & Goddard, M.E. Reconciling the analysis of IBD and IBS in complex trait studies. Nat. Rev. Genet. 11, 800–805 (2010).
Gilmour, A.R., Gogel, B.J., Cullis, B.R. & Thompson, R. ASReml User Guide Release 2.0 (VSN International, Hemel Hempstead, UK, 2006).
Yang, J., Lee, S.H., Goddard, M.E. & Visscher, P.M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Lee, S.H. & Van der Werf, J.H.J. An efficient variance component approach implementing an average information REML suitable for combined LD and linkage mapping with a general complex pedigree. Genet. Sel. Evol. 38, 25–43 (2006).
Self, S.G. & Liang, K.Y. Asymptotic properties of maximum-likelihood estimators and likelihood ratio tests under nonstandard conditions. J. Am. Stat. Assoc. 82, 605–610 (1987).
Raychaudhuri, S. et al. Accurately assessing the risk of schizophrenia conferred by rare copy-number variation affecting genes with brain function. PLoS Genet. 6, e1001097 (2010).
Acknowledgements
We thank S.D. Gordon for technical assistance. We acknowledge funding from the Australian National Health and Medical Research Council (389892, 442915, 496688, 613672 and 613601), the Australian Research Council (DP0770096, DP1093502 and FT0991360) and the US National Institute of Mental Health (MH085812). This research utilized the Cluster Computer, which is funded by the Netherlands Scientific Organization (NWO; 480-05-003). Acknowledgments for PGC-SCZ are listed in the Supplementary Note.
Author information
Authors and Affiliations
Consortia
Contributions
N.R.W. and P.M.V. devised the study. S.H.L. performed all preliminary analyses on the ISC sample and final analyses on the PGC-SCZ samples. T.R.D. performed preliminary analyses on the MGS sample. M.C.K. directed preliminary analyses on the MGS sample. S.R. undertook the quality control analysis and imputation of the PGC-SCZ samples. M.E.G. and J.Y. advised on analyses and their interpretation. P.F.S. provided interpretation in the context of schizophrenia research. N.R.W., S.H.L. and P.M.V. wrote the first draft of the manuscript. All authors contributed to the final manuscript. The ISC, MGS and PGC-SCZ members collected and genotyped cases and controls.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A full list of members is provided in the Supplementary Note.
A full list of members is provided in the Supplementary Note.
A full list of members is provided in the Supplementary Note.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–6, Supplementary Figures 1 and 2 and Supplementary Note (PDF 389 kb)
Rights and permissions
About this article
Cite this article
Lee, S., DeCandia, T., Ripke, S. et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet 44, 247–250 (2012). https://doi.org/10.1038/ng.1108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.1108
This article is cited by
-
A method for an unbiased estimate of cross-ancestry genetic correlation using individual-level data
Nature Communications (2023)
-
The genetic architecture of schizophrenia: review of large-scale genetic studies
Journal of Human Genetics (2023)
-
Magical thinking in individuals with high polygenic risk for schizophrenia but no non-affective psychoses—a general population study
Molecular Psychiatry (2022)
-
A cognitive neurogenetic approach to uncovering the structure of executive functions
Nature Communications (2022)
-
A genetic risk score using human chromosomal-scale length variation can predict schizophrenia
Scientific Reports (2021)