Abstract
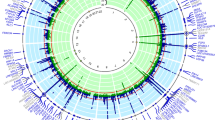
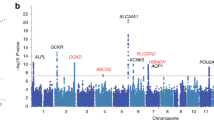
Uric acid is the end product of purine metabolism in humans and great apes, which have lost hepatic uricase activity, leading to uniquely high serum uric acid concentrations (200–500 μM) compared with other mammals (3–120 μM)1. About 70% of daily urate disposal occurs via the kidneys, and in 5–25% of the human population, impaired renal excretion leads to hyperuricemia2. About 10% of people with hyperuricemia develop gout, an inflammatory arthritis that results from deposition of monosodium urate crystals in the joint. We have identified genetic variants within a transporter gene, SLC2A9, that explain 1.7–5.3% of the variance in serum uric acid concentrations, following a genome-wide association scan in a Croatian population sample. SLC2A9 variants were also associated with low fractional excretion of uric acid and/or gout in UK, Croatian and German population samples. SLC2A9 is a known fructose transporter3, and we now show that it has strong uric acid transport activity in Xenopus laevis oocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Johnson, R.J., Titte, S., Cade, J.R., Rideout, B.A. & Oliver, W.J. Uric acid, evolution and primitive cultures. Semin. Nephrol. 25, 3–8 (2005).
Becker, M.A. & Jolly, M. Hyperuricemia and associated diseases. Rheum. Dis. Clin. North Am. 32, 275–293 (2006).
Manolescu, A.R., Augustin, R., Moley, K. & Cheeseman, C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol. Membr. Biol. 24, 455–463 (2007).
Campbell, H. et al. Effects of genome-wide heterozygosity on a range of biomedically relevant human quantitative traits. Hum. Mol. Genet. 16, 233–241 (2007).
Aulchenko, Y.S., de Koning, D.J. & Haley, C. Genomewide rapid association using mixed model and regression: a fast and simple method for genomewide pedigree-based quantitative trait loci association analysis. Genetics 177, 577–585 (2007).
Graessler, J. et al. Association of the human urate transporter 1 with reduced renal uric acid excretion and hyperuricemia in a German Caucasian population. Arthritis Rheum. 54, 292–300 (2006).
Grundy, S.M., Brewer, H.B. Jr., Cleeman, J.I., Smith, S.C. Jr. & Lenfant, C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109, 433–438 (2004).
Choi, H.K. & Ford, E.S. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am. J. Med. 120, 442–447 (2007).
Ford, E.S., Li, C., Cook, S. & Choi, H.K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 115, 2526–2532 (2007).
Seatter, M.J., De la Rue, S.A., Porter, L.M. & Gould, G.W. QLS motif in transmembrane helix VII of the glucose transporter family interacts with the C-1 position of D-glucose and is involved in substrate selection at the exofacial binding site. Biochemistry 37, 1322–1326 (1998).
Uldry, M. & Thorens, B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 447, 480–489 (2004).
Phay, J.E., Hussain, H.B. & Moley, J.F. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9). Genomics 66, 217–220 (2000).
Augustin, R. et al. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J. Biol. Chem. 279, 16229–16236 (2004).
Keembiyehetty, C. et al. Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol. Endocrinol. 20, 686–697 (2006).
Richardson, S. et al. Molecular characterization and partial cDNA cloning of facilitative glucose transporters expressed in human articular chondrocytes; stimulation of 2-deoxyglucose uptake by IGF-I and elevated MMP-2 secretion by glucose deprivation. Osteoarthritis Cartilage 11, 92–101 (2003).
Enomoto, A. & Endou, H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin. Exp. Nephrol. 9, 195–205 (2005).
Hagos, Y., Stein, D., Ugele, B., Burckhardt, G. & Bahn, A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J. Am. Soc. Nephrol. 18, 430–439 (2007).
Mount, D.B., Kwon, C.Y. & Zandi-Nejad, K. Renal urate transport. Rheum. Dis. Clin. North Am. 32, 313–331 (2006).
Heinig, M. & Johnson, R.J. Role of uric acid in hypertension, renal disease, and metabolic syndrome. Cleve. Clin. J. Med. 73, 1059–1064 (2006).
Emmerson, B.T. Effect of oral fructose on urate production. Ann. Rheum. Dis. 33, 276–280 (1974).
Rutledge, A.C. & Adeli, K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr. Rev. 65, S13–S23 (2007).
Li, S. et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 3, e194 (2007).
Wallace, C. et al. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am. J. Hum. Genet. 82, 139–149 (2008).
Rudan, I. et al. Effects of inbreeding, endogamy, genetic admixture, and outbreeding on human health: a (1001 Dalmatians) study. Croat. Med. J. 47, 601–610 (2006).
Kimber, C.H. et al. TCF7L2 in the Go-DARTS study: evidence for a gene dose effect on both diabetes susceptibility and control of glucose levels. Diabetologia 50, 1186–1191 (2007).
Morris, A.D. et al. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus. The DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland. Medicines Monitoring Unit. Lancet 350, 1505–1510 (1997).
Wallace, S.L. et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 20, 895–900 (1977).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Gray, N.K., Coller, J.M., Dickson, K.S. & Wickens, M. Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J. 19, 4723–4733 (2000).
Gabriel, S.B. et al. The structure of haplotype blocks in the human genome. Science 296, 2225–2229 (2002).
Acknowledgements
This research was funded by grants from the Medical Research Council (UK), the Wellcome Trust, Arthritis Research Campaign (S.H.R.), and Cancer Research UK (A.T.), an MRC Senior Non-Clinical Fellowship (N.K.G.), and Republic of Croatia Ministry of Science, Education and Sports grants to I.R. (108-1080315-0302), P.R. (196-1962766-2751), B.J. (196-1962766-2763) and N.S.-N. (196-1962766-2747). The Croatian and Scottish (Orcadian) groups are now components of the EU Framework 6 project EUROSPAN (Contract No. LSHG-CT-2006-018947) (I.R., H.C., J.F.W., A.F.W., N.D.H.). We thank G.W. Gould, C.I. Cheeseman and I. White for helpful discussions and advice. We acknowledge the Wellcome Trust Clinical Research Facility (Edinburgh) for performing DNA extractions (Orkney) and the genome-wide association scan, J. Ireland for computing support and C. Nicol for the figures. Special thanks to K. Wilson and R. Bisset for administrative support.
Author information
Authors and Affiliations
Contributions
H.C., I.R., N.D.H. and A.F.W. designed the study and wrote the paper. V.V., C.H., J.F., S.A.K., A.T. and P.M.M. performed statistical analyses. S.C. and J.M. carried out the genotyping. N.K.G., X.S., B.G., W.A.R. and P.H. made constructs and performed transporter assays. P.R., I.K., O.P., B.J., N.S.-N., Z.B., L.B.-L., M.P., I.M.K., L.Z. and T.S.-J. performed field work and constructed genealogies in Croatia. J.F.W. and S.H.W. provided replication in Orcadian samples. J.G., M.A., P.L.R., S.H.R., A.M. and L.D.F. provided gout and FEUA samples. C.N.A.P., C.H.K., L.A.D. and A.D.M. genotyped and analyzed the Go-DARTS dataset.
Corresponding author
Ethics declarations
Competing interests
We have filed a patent application based on this work.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Tables 1–4 (PDF 90 kb)
Rights and permissions
About this article
Cite this article
Vitart, V., Rudan, I., Hayward, C. et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40, 437–442 (2008). https://doi.org/10.1038/ng.106
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.106
This article is cited by
-
Uric acid–driven NLRP3 inflammasome activation triggers lens epithelial cell senescence and cataract formation
Cell Death Discovery (2024)
-
Lactiplantibacillus plantarum enables blood urate control in mice through degradation of nucleosides in gastrointestinal tract
Microbiome (2023)
-
Alterations in lipidome profiles distinguish early-onset hyperuricemia, gout, and the effect of urate-lowering treatment
Arthritis Research & Therapy (2023)
-
Recurrent exercise-induced acute kidney injury associated with hypouricemia: a case report and literature review
BMC Nephrology (2023)
-
GLUT9 as a potential drug target for chronic kidney disease: Drug target validation by a Mendelian randomization study
Journal of Human Genetics (2023)