Abstract
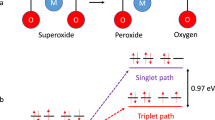
Non-aqueous metal–oxygen batteries depend critically on the reversible formation/decomposition of metal oxides on cycling. Irreversible parasitic reactions cause poor rechargeability, efficiency, and cycle life, and have predominantly been ascribed to the reactivity of reduced oxygen species with cell components. These species, however, cannot fully explain the side reactions. Here we show that singlet oxygen forms at the cathode of a lithium–oxygen cell during discharge and from the onset of charge, and accounts for the majority of parasitic reaction products. The amount increases during discharge, early stages of charge, and charging at higher voltages, and is enhanced by the presence of trace water. Superoxide and peroxide appear to be involved in singlet oxygen generation. Singlet oxygen traps and quenchers can reduce parasitic reactions effectively. Awareness of the highly reactive singlet oxygen in non-aqueous metal–oxygen batteries gives a rationale for future research towards achieving highly reversible cell operation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Larcher, D. & Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2014).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Luntz, A. C. & McCloskey, B. D. Nonaqueous Li–air batteries: a status report. Chem. Rev. 114, 11721–11750 (2014).
Choi, N.-S. et al. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 51, 9994–10024 (2012).
Lu, Y.-C. et al. Lithium-oxygen batteries: bridging mechanistic understanding and battery performance. Energy Environ. Sci. 6, 750–768 (2013).
Ren, X. & Wu, Y. A low-overpotential potassium–oxygen battery based on potassium superoxide. J. Am. Chem. Soc. 135, 2923–2926 (2013).
Walker, W. et al. A rechargeable Li–O2 battery using a lithium nitrate/N,N-dimethylacetamide electrolyte. J. Am. Chem. Soc. 135, 2076–2079 (2013).
Lim, H.-D. et al. Rational design of redox mediators for advanced Li–O2 batteries. Nat. Energy 1, 16066 (2016).
Bender, C. L., Hartmann, P., Vračar, M., Adelhelm, P. & Janek, J. On the thermodynamics, the role of the carbon cathode, and the cycle life of the sodium superoxide (NaO2) battery. Adv. Energy Mater. 4, 1301863 (2014).
Laoire, C. O., Mukerjee, S., Plichta, E. J., Hendrickson, M. A. & Abraham, K. M. Rechargeable lithium/TEGDME-LiPF6/O2 battery. J. Electrochem. Soc. 158, A302–A308 (2011).
Hassoun, J., Croce, F., Armand, M. & Scrosati, B. Investigation of the O2 electrochemistry in a polymer electrolyte solid-state cell. Angew. Chem. Int. Ed. 50, 2999–3002 (2011).
Younesi, R. et al. Ether based electrolyte, LiB(CN)4 salt and binder degradation in the Li–O2 battery studied by hard X-ray photoelectron spectroscopy (haxpes). J. Phys. Chem. C 116, 18597–18604 (2012).
Amanchukwu, C. V., Harding, J. R., Shao-Horn, Y. & Hammond, P. T. Understanding the chemical stability of polymers for lithium-air batteries. Chem. Mater. 27, 550–561 (2015).
Bryantsev, V. S. et al. The identification of stable solvents for nonaqueous rechargeable Li–air batteries. J. Electrochem. Soc. 160, A160–A171 (2013).
Xu, W. et al. The stability of organic solvents and carbon electrode in nonaqueous Li–O2 batteries. J. Power Sources 215, 240–247 (2012).
Ottakam Thotiyl, M. M. et al. A stable cathode for the aprotic Li–O2 battery. Nat. Mater. 12, 1050–1056 (2013).
Adams, B. D. et al. Towards a stable organic electrolyte for the lithium oxygen battery. Adv. Energy Mater. 5, 1400867 (2015).
Khetan, A., Luntz, A. & Viswanathan, V. Trade-offs in capacity and rechargeability in nonaqueous Li–O2 batteries: solution-driven growth versus nucleophilic stability. J. Phys. Chem. Lett. 6, 1254–1259 (2015).
Aetukuri, N. B. et al. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries. Nat. Chem. 7, 50–56 (2015).
Black, R. et al. Screening for superoxide reactivity in Li–O2 batteries: effect on Li2O2/LiOH crystallization. J. Am. Chem. Soc. 134, 2902–2905 (2012).
McCloskey, B. D. et al. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 3, 997–1001 (2012).
Younesi, R., Hahlin, M., Björefors, F., Johansson, P. & Edström, K. Li–O2 battery degradation by lithium peroxide (Li2O2): a model study. Chem. Mater. 25, 77–84 (2012).
McCloskey, B. D. et al. Limitations in rechargeability of Li–O2 batteries and possible origins. J. Phys. Chem. Lett. 3, 3043–3047 (2012).
McCloskey, B. D. et al. Combining accurate O2 and Li2O2 assays to separate discharge and charge stability limitations in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 4, 2989–2993 (2013).
Ottakam Thotiyl, M. M., Freunberger, S. A., Peng, Z. & Bruce, P. G. The carbon electrode in nonaqueous Li–O2 cells. J. Am. Chem. Soc. 135, 494–500 (2013).
Freunberger, S. A. et al. The lithium–oxygen battery with ether-based electrolytes. Angew. Chem. Int. Ed. 50, 8609–8613 (2011).
Carboni, M., Marrani, A. G., Spezia, R. & Brutti, S. 1,2-dimethoxyethane degradation thermodynamics in Li–O2 redox environments. Chem. Eur. J. 22, 17188–17203 (2016).
Wandt, J., Jakes, P., Granwehr, J., Gasteiger, H. A. & Eichel, R.-A. Singlet oxygen formation during the charging process of an aprotic lithium–oxygen battery. Angew. Chem. Int. Ed. 55, 6892–6895 (2016).
Alfano, A. J. & Christe, K. O. Singlet delta oxygen production from a gas–solid reaction. Angew. Chem. Int. Ed. 41, 3252–3254 (2002).
Li, Q. et al. A spectroscopic study on singlet oxygen production from different reaction paths using solid inorganic peroxides as starting materials. Bull. Korean Chem. Soc. 28, 1656–1660 (2007).
Schweitzer, C. & Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 103, 1685–1758 (2003).
Ogilby, P. R. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 39, 3181–3209 (2010).
Aurbach, D., McCloskey, B. D., Nazar, L. F. & Bruce, P. G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 1, 16128 (2016).
Bryantsev, V. S. et al. Predicting solvent stability in aprotic electrolyte Li–air batteries: nucleophilic substitution by the superoxide anion radical (O2·−). J. Phys. Chem. A 115, 12399–12409 (2011).
Bryantsev, V. S. & Blanco, M. Computational study of the mechanisms of superoxide-induced decomposition of organic carbonate-based electrolytes. J. Phys. Chem. Lett. 2, 379–383 (2011).
Bryantsev, V. S. & Faglioni, F. Predicting autoxidation stability of ether- and amide-based electrolyte solvents for Li–air batteries. J. Phys. Chem. A 116, 7128–7138 (2012).
Khan, A. U. Direct spectral evidence of the generation of singlet molecular oxygen (1.Delta.g) in the reaction of potassium superoxide with water. J. Am. Chem. Soc. 103, 6516–6517 (1981).
Umezawa, N. et al. Novel fluorescent probes for singlet oxygen. Angew. Chem. Int. Ed. 38, 2899–2901 (1999).
Miyamoto, S., Martinez, G. R., Medeiros, M. H. G. & Di Mascio, P. Singlet molecular oxygen generated from lipid hydroperoxides by the russell mechanism: studies using 18O-labeled linoleic acid hydroperoxide and monomol light emission measurements. J. Am. Chem. Soc. 125, 6172–6179 (2003).
Young, R. H. & Brewer, D. R. in Singlet Oxygen. Reactions with Organic Compounds Polymers (eds Ranby, B. & Rabek, J. F. ) (Wiley, 1978).
Enko, B. et al. Singlet oxygen-induced photodegradation of the polymers and dyes in optical sensing materials and the effect of stabilizers on these processes. J. Phys. Chem. A 117, 8873–8882 (2013).
Chase, G. V. et al. Soluble oxygen evolving catalysts for rechargeable metal-air batteries. US patent 13/093,759 (2011).
Chen, Y., Freunberger, S. A., Peng, Z., Fontaine, O. & Bruce, P. G. Charging a Li–O2 battery using a redox mediator. Nat. Chem. 5, 489–494 (2013).
Bryantsev, V. S., Blanco, M. & Faglioni, F. Stability of lithium superoxide LiO2 in the gas phase: computational study of dimerization and disproportionation reactions. J. Phys. Chem. A 114, 8165–8169 (2010).
Koppenol, W. H. Reactions involving singlet oxygen and the superoxide anion. Nature 262, 420–421 (1976).
Hummelshoj, J. S., Luntz, A. C. & Norskov, J. K. Theoretical evidence for low kinetic overpotentials in Li–O2 electrochemistry. J. Chem. Phys. 138, 034703 (2013).
Kang, S., Mo, Y., Ong, S. P. & Ceder, G. A facile mechanism for recharging Li2O2 in Li–O2 batteries. Chem. Mater. 25, 3328–3336 (2013).
Mo, Y., Ong, S. P. & Ceder, G. First-principles study of the oxygen evolution reaction of lithium peroxide in the lithium-air battery. Phys. Rev. B 84, 205446 (2011).
Snow, R. H. Thermodynamic Evaluation of the Possibility of Lithium Superoxide Production (Aerospace Medical Research Laboratories, 1965).
Khan, A. U. Activated oxygen: singlet molecular oxygen and superoxide anion. Photochem. Photobiol. 28, 615–626 (1978).
Borisov, S. M. et al. New NIR-emitting complexes of platinum(II) and palladium(II) with fluorinated benzoporphyrins. J. Photochem. Photobiol. A 201, 128–135 (2009).
Chen, Y., Freunberger, S. A., Peng, Z., Bardé, F. & Bruce, P. G. Li–O2 battery with a dimethylformamide electrolyte. J. Am. Chem. Soc. 134, 7952–7957 (2012).
Peng, Z., Freunberger, S. A., Chen, Y. & Bruce, P. G. A reversible and higher-rate Li–O2 battery. Science 337, 563–566 (2012).
Acknowledgements
S.A.F. is indebted to the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 636069). We further gratefully acknowledge funding from the Austrian Federal Ministry of Economy, Family and Youth and the Austrian National Foundation for Research, Technology and Development and initial funding from the Austrian Science Fund (FWF, Project No. P26870-N19). The authors thank R. Saf for help with the MS, R. Breinbauer for discussions about the reaction mechanism, S. Landgraf for help with the NIR measurement, and J. Schlegl for manufacturing instrumentation for the methods used.
Author information
Authors and Affiliations
Contributions
N.M. performed the main part of the experiments and analysed the results. B.S., S.G. and C.L. performed cell cycling, MS and NMR experiments. G.A.S. did HPLC analysis. S.A.F., D.K., C.S., O.F. and M.L. discussed the reaction mechanisms. S.M.B. supervised the optical experiments. S.A.F. conceived and directed the research, set up and performed experiments, analysed the results and wrote the manuscript with help of the other authors. All authors contributed to the discussion and interpretation of the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–15, Supplementary Table 1, Supplementary Discussion, Supplementary References (PDF 946 kb)
Rights and permissions
About this article
Cite this article
Mahne, N., Schafzahl, B., Leypold, C. et al. Singlet oxygen generation as a major cause for parasitic reactions during cycling of aprotic lithium–oxygen batteries. Nat Energy 2, 17036 (2017). https://doi.org/10.1038/nenergy.2017.36
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2017.36
This article is cited by
-
Li–air batteries: air stability of lithium metal anodes
Science China Chemistry (2024)
-
Solving the Singlet Oxygen Puzzle in Metal-O2 Batteries: Current Progress and Future Directions
Electrochemical Energy Reviews (2024)
-
Roadmap for rechargeable batteries: present and beyond
Science China Chemistry (2024)
-
Why charging Li–air batteries with current low-voltage mediators is slow and singlet oxygen does not explain degradation
Nature Chemistry (2023)
-
Li–O2 battery redox mediators go positive
Nature Chemistry (2023)