Abstract
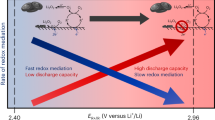
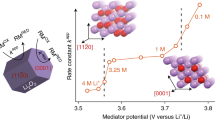
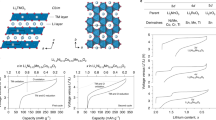
The discovery of effective catalysts is an important step towards achieving Li–O2 batteries with long cycle life and high round-trip efficiency. Soluble-type catalysts or redox mediators (RMs) possess great advantages over conventional solid catalysts, generally exhibiting much higher efficiency. Here, we select a series of organic RM candidates as a model system to identify the key descriptor in determining the catalytic activities and stabilities in Li–O2 cells. It is revealed that the level of ionization energies, readily available parameters from a database of the molecules, can serve such a role when comparing with the formation energy of Li2O2 and the highest occupied molecular orbital energy of the electrolyte. It is demonstrated that they are critical in reducing the overpotential and improving the stability of Li–O2 cells, respectively. Accordingly, we propose a general principle for designing feasible catalysts and report a RM, dimethylphenazine, with a remarkably low overpotential and high stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Liu, T. et al. Cycling Li-O2 batteries via LiOH formation and decomposition. Science 350, 530–533 (2015).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nature Mater. 11, 19–29 (2012).
Black, R. et al. Screening for superoxide reactivity in Li-O2 batteries: effect on Li2O2/LiOH crystallization. J. Am. Chem. Soc. 134, 2902–2905 (2012).
Lim, H.-D. et al. Enhanced power and rechargeability of a Li−O2 battery based on a hierarchical-fibril CNT electrode. Adv. Mater. 25, 1348–1352 (2013).
Xu, J.-J. et al. Tailoring deposition and morphology of discharge products towards high-rate and long-life lithium-oxygen batteries. Nature Commun. 4, 2438 (2013).
Lu, J. et al. Aprotic and aqueous Li–O2 batteries. Chem. Rev. 114, 5611–5640 (2014).
Li, F. et al. The water catalysis at oxygen cathodes of lithium-oxygen cells. Nature Commun. 6, 7843 (2015).
Balaish, M., Kraytsberg, A. & Ein-Eli, Y. A critical review on lithium-air battery electrolytes. Phys. Chem. Chem. Phys. 16, 2801–2822 (2014).
Lim, H.-D. et al. The potential for long-term operation of a lithium-oxygen battery using a non-carbonate-based electrolyte. Chem. Commun. 48, 8374–8376 (2012).
Aetukuri, N. B. et al. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries. Nature Chem. 7, 50–56 (2015).
Zhu, Y. et al. MnOx decorated CeO2 nanorods as cathode catalyst for rechargeable lithium-air batteries. J. Mater. Chem. A 3, 13563–13567 (2015).
Black, R., Adams, B. & Nazar, L. F. Non-aqueous and hybrid Li-O2 batteries. Adv. Energy Mater. 2, 801–815 (2012).
Freunberger, S. A. et al. Reactions in the rechargeable lithium–O2 battery with alkyl carbonate electrolytes. J. Am. Chem. Soc. 133, 8040–8047 (2011).
McCloskey, B. D. et al. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 3, 997–1001 (2012).
Liu, S. et al. Free-standing, hierarchically porous carbon nanotube film as a binder-free electrode for high-energy Li-O2 batteries. J. Mater. Chem. A 1, 12033–12037 (2013).
Cheng, F. & Chen, J. Lithium-air batteries: something from nothing. Nature Chem. 4, 962–963 (2012).
Suntivich, J. et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nature Chem. 3, 546–550 (2011).
Lu, Y.-C. et al. Platinum–gold nanoparticles: a highly active bifunctional electrocatalyst for rechargeable lithium–air batteries. J. Am. Chem. Soc. 132, 12170–12171 (2010).
Peng, Z., Freunberger, S. A., Chen, Y. & Bruce, P. G. A reversible and higher-rate Li-O2 battery. Science 337, 563–566 (2012).
Ryu, W.-H. et al. Bifunctional composite catalysts using Co3O4 nanofibers immobilized on nonoxidized graphene nanoflakes for high-capacity and long-cycle Li–O2 batteries. Nano Lett. 13, 4190–4197 (2013).
McCloskey, B. D. et al. On the efficacy of electrocatalysis in nonaqueous Li–O2 batteries. J. Am. Chem. Soc. 133, 18038–18041 (2011).
Chen, Y. et al. Charging a Li–O2 battery using a redox mediator. Nature Chem. 5, 489–494 (2013).
Lim, H.-D. et al. Superior rechargeability and efficiency of lithium–oxygen batteries: hierarchical air electrode architecture combined with a soluble catalyst. Angew. Chem. Int. Ed. 53, 3926–3931 (2014).
Chase, G. V. et al. Soluble oxygen evolving catalysts for rechargeable metal-air batteries. US patent US2011/033821 (2011).
Wang, Y. & Xia, Y. Li-O2 batteries: an agent for change. Nature Chem. 5, 445–447 (2013).
Yu, M., Ren, X., Ma, L. & Wu, Y. Integrating a redox-coupled dye-sensitized photoelectrode into a lithium–oxygen battery for photoassisted charging. Nature Commun. 5, 5111 (2014).
Bergner, B. J. et al. TEMPO: a mobile catalyst for rechargeable Li-O2 batteries. J. Am. Chem. Soc. 136, 15054–15064 (2014).
Sun, D. et al. A solution-phase bifunctional catalyst for lithium–oxygen batteries. J. Am. Chem. Soc. 136, 8941–8946 (2014).
Paduszek, B. & Kalinowski, M. K. Redox behaviour of phenothiazine and phenazine in organic solvents. Electrochim. Acta 28, 639–642 (1983).
Ionization energy evaluation in NIST Chemistry WebBook NIST Standard Reference Database Number 69 (NIST, 2016); http://webbook.nist.gov
Cederbaum, L. S. & Domcke, W. Theoretical aspects of ionization potentials and photoelectron spectroscopy: a Green’s function approach. Adv. Chem. Phys. 39, 205–344 (1977).
Gleiter, R. et al. Photoelectron and electronic absorption spectra of tetrathiafulvalene and related compounds. Ber. Bunsenges. Phys. Chem. 79, 1218–1226 (1975).
Rabalais, J. W. et al. Electron spectroscopy of open-shell systems: spectra of Ni(C5H5)2, Fe(C5H5)2, Mn(C5H5)2, and Cr(C5H5)2 . J. Chem. Phys. 57, 1185–1192 (1972).
Foster, R. Ionization potentials of electron donors. Nature 183, 1253 (1959).
Maier, J. P. Photoelectron spectroscopy of peri-amino naphthalenes. Helv. Chim. Acta 57, 994–1003 (1974).
Egdell, R., Green, J. C. & Rao, C. N. R. Photoelectron spectra of substituted benzenes. Chem. Phys. Lett. 33, 600–607 (1975).
Rozeboom, M. D., Houk, K. N., Searles, S. & Seyedrezai, S. E. Photoelectron spectroscopy of N-aryl cyclic amines. Variable conformations and relationships to gas- and solution-phase basicities. J. Am. Chem. Soc. 104, 3448–3453 (1982).
Matsen, F. A. Electron affinities, methyl affinities, and ionization energies of condensed ring aromatic hydrocarbons. J. Chem. Phys. 24, 602–606 (1956).
Fraser-Monteiro, M. L. et al. Thermochemistry and dissociation dynamics of state-selected C4H8O2+ ions. 1. 1,4-dioxane. J. Phys. Chem. 86, 739–747 (1982).
Watanabe, K., Nakayama, T. & Mottl, J. Ionization potentials of some molecules. J. Quant. Spectrosc. Radiat. Transfer 2, 369–382 (1962).
Chen, Y., Shen, L. & Li, X. Effects of heteroatoms of tetracene and pentacene derivatives on their stability and singlet fission. J. Phys. Chem. A 118, 5700–5708 (2014).
Liang, Z. et al. Unexpected photooxidation of H-bonded tetracene. Org. Lett. 10, 2007–2010 (2008).
Khetan, A., Pitsch, H. & Viswanathan, V. Identifying descriptors for solvent stability in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 5, 1318–1323 (2014).
Khetan, A., Pitsch, H. & Viswanathan, V. Solvent degradation in nonaqueous Li-O2 batteries: oxidative stability versus H-abstraction. J. Phys. Chem. Lett. 5, 2419–2424 (2014).
McCloskey, B. D. et al. Limitations in rechargeability of Li-O2 batteries and possible origins. J. Phys. Chem. Lett. 3, 3043–3047 (2012).
Bryantsev, V. S. et al. The identification of stable solvents for nonaqueous rechargeable Li-air batteries. J. Electrochem. Soc. 160, A160-A171 (2013).
Bryantsev, V. S. Predicting the stability of aprotic solvents in Li-air batteries: pKa calculations of aliphatic C–H acids in dimethyl sulfoxide. Chem. Phys. Lett. 558, 42–47 (2013).
Bryantsev, V. S. et al. Predicting solvent stability in aprotic electrolyte Li–air batteries: nucleophilic substitution by the superoxide anion radical (O2⋅−). J. Phys. Chem. A 115, 12399–12409 (2011).
Garcia-Lastra, J. M., Rostgaard, C., Rubio, A. & Thygesen, K. S. Polarization-induced renormalization of molecular levels at metallic and semiconducting surfaces. Phys. Rev. B 80, 245427 (2009).
Johnson, P. D. & Hulbert, S. L. Inverse-photoemission studies of adsorbed diatomic molecules. Phys. Rev. B 35, 9427–9436 (1987).
Repp, J. et al. Molecules on insulating films: scanning-tunneling microscopy imaging of individual molecular orbitals. Phys. Rev. Lett. 94, 026803 (2005).
Kundu, D., Black, R., Adams, B. & Nazar, L. F. A highly active low voltage redox mediator for enhanced rechargeability of lithium–oxygen batteries. ACS Cent. Sci. 1, 510–515 (2015).
Takimiya, K., Yamamoto, T., Ebata, H. & Izawa, T. Design strategy for air-stable organic semiconductors applicable to high-performance field-effect transistors. Sci. Technol. Adv. Mater. 8, 273–276 (2007).
Nelson, R. F., Leedy, D. W., Seo, E. T. & Adams, R. Anodic oxidation of 5,10-dihydro-5,10-dimethylphenazine. Z. Anal. Chem. 224, 184–196 (1966).
Adams, B. D. et al. Current density dependence of peroxide formation in the Li-O2 battery and its effect on charge. Energy Environ. Sci. 6, 1772–1778 (2013).
Frisch, M. et al. Gaussian 09 Revision D 1 (Gaussian, 2009).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Schäfer, A., Huber, C. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994).
Schäfer, A., Horn, H. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 97, 2571–2577 (1992).
Klaumünzer, B., Kröner, D. & Saalfrank, P. (TD-)DFT calculation of vibrational and vibronic spectra of riboflavin in solution. J. Phys. Chem. B 114, 10826–10834 (2010).
Trasatti, S. The absolute electrode potential: an explanatory note (Recommendations 1986). Pure Appl. Chem. 58, 955–966 (1986).
Gritzner, G. Standard electrode potentials of M + |M couples in non-aqueous solvents (molecular liquids). J. Mol. Liq. 156, 103–108 (2010).
Cramer, C. J. Essentials of Computational Chemistry: Theories and Models 2nd edn (Wiley, 2014).
Acknowledgements
This work was supported by the HMC (Hyundai Motor Company), the Project Code (IBS-R006-G1), and the National Research Foundation of Korea(NRF) grant funded by the Korean Government(MSIP) (No. 2015R1A2A1A10055991). Y.Z. and K.C. acknowledge the Global Frontier R&D Program on Center for Multiscale Energy System (NRF-2011-0031571).
Author information
Authors and Affiliations
Contributions
H.-D.L. and Y.Z. designed the experiments with help from J.H., J.K., H.G., Y.K., M.L. and K.C. The computations were performed by B.L. The original idea was conceived by K.K., who supervised all the experiments and calculations. All the authors discussed the results and contributed to preparing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–18, Supplementary Tables 1–2, Supplementary Notes 1–9, Supplementary References. (PDF 2205 kb)
Rights and permissions
About this article
Cite this article
Lim, HD., Lee, B., Zheng, Y. et al. Rational design of redox mediators for advanced Li–O2 batteries. Nat Energy 1, 16066 (2016). https://doi.org/10.1038/nenergy.2016.66
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.66
This article is cited by
-
Supercharged supramolecular binding constants
Nature Chemical Engineering (2024)
-
Triarylmethyl cation redox mediators enhance Li–O2 battery discharge capacities
Nature Chemistry (2023)
-
Why charging Li–air batteries with current low-voltage mediators is slow and singlet oxygen does not explain degradation
Nature Chemistry (2023)
-
Building Better Full Manganese-Based Cathode Materials for Next-Generation Lithium-Ion Batteries
Electrochemical Energy Reviews (2023)
-
Addressing Transport Issues in Non-Aqueous Li–air Batteries to Achieving High Electrochemical Performance
Electrochemical Energy Reviews (2023)