Abstract
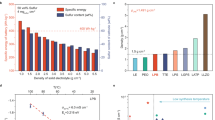
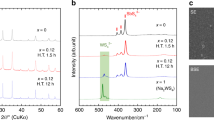
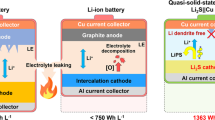
Compared with lithium-ion batteries with liquid electrolytes, all-solid-state batteries offer an attractive option owing to their potential in improving the safety and achieving both high power and high energy densities. Despite extensive research efforts, the development of all-solid-state batteries still falls short of expectation largely because of the lack of suitable candidate materials for the electrolyte required for practical applications. Here we report lithium superionic conductors with an exceptionally high conductivity (25 mS cm−1 for Li9.54Si1.74P1.44S11.7Cl0.3), as well as high stability ( ∼0 V versus Li metal for Li9.6P3S12). A fabricated all-solid-state cell based on this lithium conductor is found to have very small internal resistance, especially at 100 ∘C. The cell possesses high specific power that is superior to that of conventional cells with liquid electrolytes. Stable cycling with a high current density of 18 C (charging/discharging in just three minutes; where C is the C-rate) is also demonstrated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nature Mater. 7, 845–854 (2008).
Scrosati, B. & Garche, J. Lithium batteries: status, prospects and future. J. Power Sources 195, 2419–2430 (2010).
Goodenough, J. Rechargeable batteries: challenges old and new. J. Solid State Electrochem. 16, 2019–2029 (2012).
Winter, M. & Brodd, R. J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 104, 4245–4269 (2004).
Robinson, A. L. & Janek, J. Solid-state batteries enter EV fray. MRS Bull. 39, 1046–1047 (2014).
Seino, Y. et al. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 7, 627–631 (2014).
Kamaya, N. et al. A lithium superionic conductor. Nature Mater. 10, 682–686 (2011).
Wang, Y. et al. Design principles for solid-state lithium superionic conductors. Nature Mater. 14, 1026-1031 (2015).
Ohtomo, T. et al. All-solid-state lithium secondary batteries using the 75Li2S⋅25P2S5 glass and the 70Li2S⋅30P2S5 glass-ceramic as solid electrolytes. J. Power Sources 233, 231–235 (2013).
Kwon, O. et al. Synthesis, structure, and conduction mechanism of the lithium superionic conductor Li10+δGe1+δP2−δS12 . J. Mater. Chem. A 3, 438–446 (2015).
Hori, S. et al. Structure–property relationships in lithium superionic conductors having a Li10GeP2S12-type structure. Acta Crystallogr. B B71, 727–736 (2015).
Kanno, R. et al. A self-assembled breathing interface for all-solid-state ceramic lithium batteries. Electrochem. Solid-State Lett. 7, A455-A458 (2004).
Bron, P. et al. Li10SnP2S12: an affordable lithium superionic conductor. J. Am. Chem. Soc. 135, 15694–15697 (2013).
Kuhn, A. et al. A new ultrafast superionic Li-conductor: ion dynamics in Li11Si2PS12 and comparison with other tetragonal LGPS-type electrolytes. Phys. Chem. Chem. Phys. 16, 14669–14674 (2014).
Hori, S. et al. Synthesis, structure, and ionic conductivity of solid solution, Li10+δ M1+δP2−δS12 (M = Si, Sn). Faraday Discuss. 176, 83–94 (2014).
Huang, W. & Frech, R. Vibrational spectroscopic and electrochemical studies of the low and high temperature phases of LiCo1−xMxO2 (M = Ni or Ti). Solid State Ion. 86–88, 395–400 (1996).
Ohta, S., Kobayashi, T., Seki, J. & Asaoka, T. Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte. J. Power Sources 202, 332–335 (2012).
Aoki, K., Baars, A., Jaworski, A. & Osteryoung, J. Chronoamperometry of strong acids without supporting electrolyte. J. Electroanal. Chem. 472, 1–6 (1999).
Wetjen, M., Kim, G.-T., Joost, M., Winter, M. & Passerini, S. Temperature dependence of electrochemical properties of cross-linked poly(ethylene oxide)–lithium bis(trifluoromethanesulfonyl)imide–N-butyl-N-methylpyrrolidinium bis (trifluoromethanesulfonyl) imide solid polymer electrolytes for lithium batteries. Electrochim. Acta 87, 779–787 (2013).
Capiglia, C. et al. 7Li and 19F diffusion coefficients and thermal properties of non-aqueous electrolyte solutions for rechargeable lithium batteries. J. Power Sources 81–82, 859–862 (1999).
Abe, T., Fukuda, H., Iriyama, Y. & Ogumi, Z. Solvated Li-ion transfer at interface between graphite and electrolyte. J. Electrochem. Soc. 151, A1120–A1123 (2004).
Abe, T., Sagane, F., Ohtsuka, M., Iriyama, Y. & Ogumi, Z. Lithium-ion transfer at the interface between lithium-ion conductive ceramic electrolyte and liquid electrolyte—A key to enhancing the rate capability of lithium-ion batteries. J. Electrochem. Soc. 152, A2151–A2154 (2005).
Choi, D. et al. Li-ion batteries from LiFePO4 cathode and anatase/graphene composite anode for stationary energy storage. Electrochem. Commun. 12, 378–381 (2010).
Lee, S. W. et al. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nature Nanotech. 5, 531–537 (2010).
Nagasubramanian, G. Electrical characteristics of 18650 Li-ion cells at low temperatures. J. Appl. Electrochem. 31, 99–104 (2001).
Cericola, D., Novák, P., Wokaun, A. & Köttz, R. Hybridization of electrochemical capacitors and rechargeable batteries: an experimental analysis of the different possible approaches utilizing activated carbon, Li4Ti5O12 and LiMn2O4 . J. Power Sources 196, 10305–10313 (2011).
Khomenko, V., Raymundo-Piñero, E. & Béguin, F. A new type of high energy asymmetric capacitor with nanoporous carbon electrodes in aqueous electrolyte. J. Power Sources 195, 4234–4241 (2010).
Zhang, J., Jiang, J., Li, H. & Zhao, X. S. A high-performance asymmetric supercapacitor fabricated with graphene-based electrodes. Energy Environ. Sci. 4, 4009–4015 (2011).
Gallant, B. M. et al. The Lithium Air Battery 121–158 (Springer, 2014).
Peng, H.-J. et al. Nanoarchitectured graphene/CNT@porous carbon with extraordinary electrical conductivity and interconnected micro/mesopores for lithium-sulfur batteries. Adv. Funct. Mater. 24, 2772–2781 (2014).
Imamura, D., Miyama, M., Hibino, M. & Kubo, T. Mg intercalation properties into V2O5 gel/carbon composites under high-rate condition. J. Electrochem. Soc. 150, A753–A758 (2003).
Lin, M.-C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 324–328 (2015).
Li, S. et al. Effect of carbon matrix dimensions on the electrochemical properties of Na3V2(PO4)3 nanograins for high-performance symmetric sodium-ion batteries. Adv. Mater. 26, 3545–3553 (2014).
Ohta, N. et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater. 18, 2226–2229 (2006).
Maier, J. Nanoionics: ion transport and electrochemical storage in confined systems. Nature Mater. 4, 806–815 (2005).
Oishi, R. et al. Rietveld analysis software for J-PARC. Nucl. Instrum. Methods Phys. Res. A 600, 94–96 (2009).
Ishikawa, Y. et al. Z-MEM & Z-3D, maximum entropy method and visualization software for electron/nuclear density distribution in Z-Code. In ICANS XXI DAA-P08 (J-PARC, 2014).
Sakata, M. & Sato, M. Accurate structure analysis by the maximum-entropy method. Acta Crystallogr. A 46, 263–270 (1990).
Ishikawa, Y., Yonemura, M. & Kamiyama, T. Z-3D, Textbook of Z-code Powder Diffraction Data Analysis School (KEK, 2014).
Ohta, N. et al. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 9, 1486–1490 (2007).
Acknowledgements
The authors thank T. Yabutani, R. Saito and K. Mukoyama for their support in the preparation of the all-solid-state and lithium-ion cells. They also thank H. Hirokawa for his support in the synthesis of Li9.6P3S12. This study was supported by the Post-LiEAD project of the New Energy and Industry Technology Development Organization (NEDO), Japan. The synchrotron radiation experiments were carried out as projects approved by the Japan Synchrotron Radiation Institute (JASRI) (proposal No. 2014A1408 and 2014A1763). The neutron radiation experiments were performed at the Japan Proton Accelerator Research Complex (J-PARC) (proposal No. 2014AM1004, 2014BM0006 and 2014BM0012).
Author information
Authors and Affiliations
Contributions
Y.K. and S.H. designed and conducted the experimental work. Y.K., S.H., T.S., K.S., M.H. and R.K. analysed the electrochemical data. S.H., A.M. and M.Y. measured the synchrotron X-ray and neutron diffraction of superionic conductors. Y.K., S.H., M.Y. and R.K. analysed the crystal structure. Y.K., S.H. and R.K. wrote the manuscript. H.I. and R.K. directed this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–12, Supplementary Table 1–6, Supplementary Methods and Supplementary References. (PDF 2379 kb)
Supplementary Video 1
Description of the bi-polar stacking cell system. (MP4 47865 kb)
Rights and permissions
About this article
Cite this article
Kato, Y., Hori, S., Saito, T. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat Energy 1, 16030 (2016). https://doi.org/10.1038/nenergy.2016.30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.30
This article is cited by
-
Chloride electrode composed of ubiquitous elements for high-energy-density all-solid-state sodium-ion batteries
Scientific Reports (2024)
-
Ionic liquids in green energy storage devices: lithium-ion batteries, supercapacitors, and solar cells
Monatshefte für Chemie - Chemical Monthly (2024)
-
Influence of atmospheric moisture on the gas evolution tolerance of halide solid electrolytes
Journal of Solid State Electrochemistry (2024)
-
In situ structural characterization of Li3PS4 solid electrolytes under high pressure
Journal of Solid State Electrochemistry (2024)
-
Enhanced capacity of all-solid-state battery comprising LiNbO3-coated Li(Ni0.8Co0.1Mn0.1)O2 Cathode, Li5.4(PS4)(S0.4Cl1.0Br0.6) solid electrolyte and lithium metal anode
Journal of Solid State Electrochemistry (2024)