Abstract
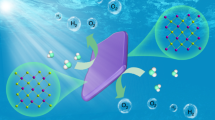
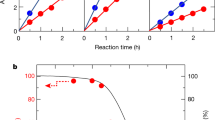
The solar-driven splitting of hydrohalic acids (HX) is an important and fast growing research direction for H2 production. In addition to the hydrogen, the resulting chemicals (X2/X3−) can be used to propagate a continuous process in a closed cycle and are themselves useful products. Here we present a strategy for photocatalytic hydrogen iodide (HI) splitting using methylammonium lead iodide (MAPbI3) in an effort to develop a cost-effective and easily scalable process. Considering that MAPbI3 is a water-soluble ionic compound, we exploit the dynamic equilibrium of the dissolution and precipitation of MAPbI3 in saturated aqueous solutions. The I− and H+ concentrations of the aqueous solution are determined to be the critical parameters for the stabilization of the tetragonal MAPbI3 phase. Stable and efficient H2 production under visible light irradiation was demonstrated. The solar HI splitting efficiency of MAPbI3 was 0.81% when using Pt as a cocatalyst.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
14 July 2017
In the PDF version of this article previously published, the year of publication provided in the footer of each page and in the 'How to cite' section was erroneously given as 2017, it should have been 2016. This error has now been corrected. The HTML version of the article was not affected.
References
Onuki, K., Kubo, S., Terada, A., Sakaba, N. & Hino, R. Thermochemical water-splitting cycle using iodine and sulfur. Energy Environ. Sci. 2, 491–497 (2009).
Beghi, G. E. Review of thermochemical hydrogen production. Int. J. Hydrog. Energy 6, 555–566 (1981).
Roth, M. & Knoche, K. F. Thermochemical water splitting through direct HI-decomposition from H2O/HI/I2 solutions. Int. J. Hydrog. Energy 14, 545–549 (1989).
Norman, J. H., Mysels, K. J., Sharp, R. & Williamson, D. Studies of the sulfur–iodine thermochemical water-splitting cycle. Int. J. Hydrog. Energy 7, 545–556 (1982).
Seifritz, W. The role of nuclear energy in the more efficient exploitation of fossil fuel resources. Int. J. Hydrog. Energy 3, 11–20 (1978).
Cerri, G. et al. Sulfur–iodine plant for large scale hydrogen production by nuclear power. Int. J. Hydrog. Energy 35, 4002–4014 (2010).
Brown, L. C., Lentsch, R. D., Besenbruch, G. E., Schultz, K. R. & Funk, J. E. Alternative Flowsheets for the Sulfur-Iodine Thermochemical Hydrogen Cycle (US Department of Energy, 2003).
Esswein, A. J. & Nocera, D. G. Hydrogen production by molecular photocatalysis. Chem. Rev. 107, 4022–4047 (2007).
Powers, D. C. et al. Photocrystallographic observation of halide-bridged intermediates in halogen photoeliminations. J. Am. Chem. Soc. 136, 15346–15355 (2014).
Levy-Clement, C., Heller, A., Bonner, W. A. & Parkinson, B. A. Spontaneous photoelectrolysis of HBr and HI. J. Electrochem. Soc. 129, 1701–1705 (1982).
Newson, J. D. & Riddiford, A. C. The kinetics of the iodine redox process at platinum electrodes. J. Electrochem. Soc. 108, 699–706 (1961).
Huskinson, B., Rugolo, J., Mondal, S. K. & Aziz, M. J. A high power density, high efficiency hydrogen–chlorine regenerative fuel cell with a low precious metal content catalyst. Energy Environ. Sci. 5, 8690–8698 (2012).
Taylor, G. R. & Butler, M. A comparison of the virucidal properties of chlorine, chlorine dioxide, bromine chloride and iodine. J. Hyg. 89, 321–328 (1982).
Yeo, R. S. & Chin, D.-T. A hydrogen–bromine cell for energy storage applications. J. Electrochem. Soc. 127, 549–555 (1980).
Baglio, J. A. et al. Characterization of n-type semiconducting tungsten disulfide photoanodes in aqueous and nonaqueous electrolyte solutions photo-oxidation of halides with high efficiency. J. Electrochem. Soc. 129, 1461–1472 (1982).
Singh, N. et al. Stable electrocatalysts for autonomous photoelectrolysis of hydrobromic acid using single-junction solar cells. Energy Environ. Sci. 7, 978–981 (2014).
Powers, D. C., Hwang, S. J., Zheng, S.-L. & Nocera, D. G. Halide-bridged binuclear HX-splitting catalysts. Inorg. Chem. 53, 9122–9128 (2014).
McKone, J. R., Potash, R. A., DiSalvo, F. J. & Abruña, H. D. Unassisted HI photoelectrolysis using n-WSe2 solar absorbers. Phys. Chem. Chem. Phys. 17, 13984–13991 (2015).
Ardo, S., Park, S. H., Warren, E. L. & Lewis, N. S. Unassisted solar-driven photoelectrosynthetic HI splitting using membrane-embedded Si microwire arrays. Energy Environ. Sci. 8, 1484–1492 (2015).
Bajdich, M., García-Mota, M., Vojvodic, A., Nørskov, J. K. & Bell, A. T. Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc. 135, 13521–13530 (2013).
Mitzi, D. B., Wang, S., Feild, C. A., Chess, C. A. & Guloy, A. M. Conducting layered organic-inorganic halides containing 〈110〉-oriented perovskite sheets. Science 267, 1473–1476 (1995).
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Im, J.-H., Lee, C.-R., Lee, J.-W., Park, S.-W. & Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 3, 4088–4093 (2011).
Xing, G. et al. Long-range balanced electron-and hole-transport lengths in organic–inorganic CH3NH3PbI3 . Science 342, 344–347 (2013).
Miyata, A. et al. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 11, 582–587 (2015).
Jeon, N. J. et al. Compositional engineering of perovskite materials for high-performance solar cells. Nature 517, 476–480 (2015).
Li, X. et al. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω-ammonium chlorides. Nat. Chem. 7, 703–711 (2015).
Im, J.-H., Jang, I.-H., Pellet, N., Grätzel, M. & Park, N.-G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotech. 9, 927–932 (2014).
Liu, M., Johnston, M. B. & Snaith, H. J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501, 395–398 (2013).
Xing, G. et al. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 13, 476–480 (2014).
Tan, Z.-K. et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotech. 9, 687–692 (2014).
Dou, L. et al. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 5, 5404 (2014).
Hao, F., Stoumpos, C. C., Cao, D. H., Chang, R. P. H. & Kanatzidis, M. G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photon. 8, 489–494 (2014).
Park, B.-W. et al. Bismuth based hybrid perovksites A3Bi2I9 (A: methylammonium or cesium) for solar cell application. Adv. Mater. 27, 6806–6813 (2015).
Imler, G. H. et al. Solid state transformation of the crystalline monohydrate (CH3NH3)PbI3(H2O) to the (CH3NH3)PbI3 perovskite. Chem. Commun. 51, 11290–11292 (2015).
Christians, J. A., Miranda Herrera, P. A. & Kamat, P. V. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J. Am. Chem. Soc. 137, 1530–1538 (2015).
Clever, H. L. & Johnston, F. J. The solubility of some sparingly soluble lead salts: an evaluation of the solubility in water and aqueous electrolyte solution. J. Phys. Chem. Ref. Data 9, 751–784 (1980).
Lanford, O. E. & Kiehl, S. J. The solubility of lead iodide in solutions of potassium iodide-complex lead iodide ions. J. Am. Chem. Soc. 63, 667–669 (1941).
Gilli, P., Pretto, L., Bertolasi, V. & Gilli, G. Predicting hydrogen-bond strengths from acid-base molecular properties. The pKa slide rule: toward the solution of a long-lasting problem. Acc. Chem. Res. 42, 33–44 (2009).
Stoumpos, C. C., Malliakas, C. D. & Kanatzidis, M. G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52, 9019–9038 (2013).
Noh, J. H., Im, S. H., Heo, J. H., Mandal, T. N. & Seok, S. I. Chemical management for colorful, efficient, and stable inorganic–organic hybrid nanostructured solar cells. Nano Lett. 13, 1764–1769 (2013).
Kim, H.-S. et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2, 591 (2012).
Awtrey, A. D. & Connick, R. E. The absorption spectra of I2, I3−, I−, IO3−, S4O6= and S2O3=. Heat of the reaction I3− = I2 + I−. J. Am. Chem. Soc. 73, 1842–1843 (1951).
Griffith, R. O., McKeown, A. & Taylor, R. P. Kinetics of the reaction of iodine with hypophosphorous acid and with hypophosphites. Trans. Faraday Soc. 36, 752–766 (1940).
Tada, H. & Tanaka, M. Dependence of TiO2 photocatalytic activity upon its film thickness. Langmuir 13, 360–364 (1997).
Xiao, Z. et al. Solvent annealing of perovskite-induced crystal growth for photovoltaic-device efficiency enhancement. Adv. Mater. 26, 6503–6509 (2014).
De Wolf, S. et al. Organometallic halide perovskites: sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 5, 1035–1039 (2014).
Hisatomi, T., Maeda, K., Takanabe, K., Kubota, J. & Domen, K. Aspects of the water splitting mechanism on (Ga1−xZnx)(N1−xOx) photocatalyst modified with Rh2−yCryO3 cocatalyst. J. Phys. Chem. C 113, 21458–21466 (2009).
Acknowledgements
This research was supported by the Global Frontier R&D Program of the Center for Multiscale Energy System funded by the National Research Foundation under the Ministry of Science, ICT & Future, Korea (2012M3A6A7054855), by KIST Institutional Program (0543-20160004), and by the Ministry of Trade, Industry & Energy (MOTIE) under Industrial Strategic Technology Development Program, Korea (0417-2016-0019).
Author information
Authors and Affiliations
Contributions
K.T.N. conceived and supervised the project. S.P. conceived, synthesized and characterized the MAPbI3 inside the aqueous solution system, designed experiments and co-wrote the manuscript. W.J.C. characterized the photocatalytic reaction and co-wrote the manuscript. C.W.L. and S.P. supported analysis of the GC data of HI splitting reaction. H.-Y.A. measured the SEM image of MAPbI3 powder. The manuscript was mainly written and revised by K.T.N., S.P. and W.J.C. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–14, Supplementary Table 1 and 2, Supplementary References. (PDF 1539 kb)
Supplementary Video 1
Phase change of MAPbI3 powder (black) into MAPb(I1−xBrx)3 (orange) immediately after dipping into HBr saturated solution (transparent) because of the dynamic equilibrium between powder and saturated solution, with the powder changing colour from black to orange as the Br atoms substituted and the solution changing to yellow due to I atoms dissolving into the solution resulting in the formation of ions. (AVI 32912 kb)
Rights and permissions
About this article
Cite this article
Park, S., Chang, W., Lee, C. et al. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. Nat Energy 2, 16185 (2017). https://doi.org/10.1038/nenergy.2016.185
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.185
This article is cited by
-
Nickel Phosphide-Coupled Methylammonium Lead Halide as an Efficient and Stable Photocatalyst for Photocatalytic H2 Evolution from HI Splitting
Catalysis Letters (2024)
-
Self-powered perovskite photon-counting detectors
Nature (2023)
-
Organic polystyrene and inorganic silica double shell protected lead halide perovskite nanocrystals with high emission efficiency and superior stability
Nano Research (2023)
-
Environmentally Stable Mesoporous g-C3N4 Modified Lead-Free Double Perovskite Cs2AgBiBr6 for Highly Efficient Photocatalytic Hydrogen Evolution
Catalysis Letters (2023)
-
Efficient CO2 Reduction to Formate on CsPbI3 Nanocrystals Wrapped with Reduced Graphene Oxide
Nano-Micro Letters (2023)