Abstract
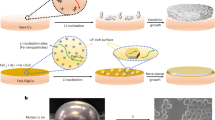
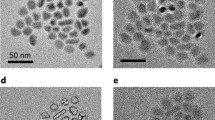
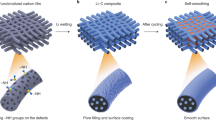
Lithium metal is an attractive anode material for rechargeable batteries, owing to its high theoretical specific capacity of 3,860 mAh g−1. Despite extensive research efforts, there are still many fundamental challenges in using lithium metal in lithium-ion batteries. Most notably, critical information such as its nucleation and growth behaviour remains elusive. Here we explore the nucleation pattern of lithium on various metal substrates and unravel a substrate-dependent growth phenomenon that enables selective deposition of lithium metal. With the aid of binary phase diagrams, we find that no nucleation barriers are present for metals exhibiting a definite solubility in lithium, whereas appreciable nucleation barriers exist for metals with negligible solubility. We thereafter design a nanocapsule structure for lithium metal anodes consisting of hollow carbon spheres with nanoparticle seeds inside. During deposition, the lithium metal is found to predominantly grow inside the hollow carbon spheres. Such selective deposition and stable encapsulation of lithium metal eliminate dendrite formation and enable improved cycling, even in corrosive alkyl carbonate electrolytes, with 98% coulombic efficiency for more than 300 cycles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Whittingham, M. S. History, evolution, and future status of energy storage. Proc. IEEE 100, 1518–1534 (2012).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nature Mater. 11, 19–29 (2012).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Whittingham, M. S. Electrical energy storage and intercalation chemistry. Science 192, 1126–1127 (1976).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 148, 405–416 (2002).
Kamaya, N. et al. A lithium superionic conductor. Nature Mater. 10, 682–686 (2011).
Bouchet, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nature Mater. 12, 452–457 (2013).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nature Commun. 6, 6362 (2015).
Zheng, G. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nature Nanotech. 9, 618–623 (2014).
Yan, K. et al. Ultrathin two-dimensional atomic crystals as stable interfacial layer for improvement of lithium metal anode. Nano Lett. 14, 6016–6022 (2014).
Bhattacharyya, R. et al. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nature Mater. 9, 504–510 (2010).
Chandrashekar, S. et al. 7Li MRI of Li batteries reveals location of microstructural lithium. Nature Mater. 11, 311–315 (2012).
Huggins, R. Advanced Batteries 123–149 (Springer, 2009).
Aurbach, D., Weissman, I., Schechter, A. & Cohen, H. X-ray photoelectron spectroscopy studies of lithium surfaces prepared in several important electrolyte solutions. A comparison with previous studies by Fourier transform infrared spectroscopy. Langmuir 12, 3991–4007 (1996).
López, C. M., Vaughey, J. T. & Dees, D. W. Morphological transitions on lithium metal anodes. J. Electrochem. Soc. 156, A726 (2009).
Aurbach, D. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 89, 206–218 (2000).
Visco, S., Nimon, Y. & Katz, B. Compositions and methods for protection of active metal anodes and polymer electrolytes. US patent 20040131944 A1 (2004).
La Mantia, F., Wessells, C. D., Deshazer, H. D. & Cui, Y. Reliable reference electrodes for lithium-ion batteries. Electrochem. Commun. 31, 141–144 (2013).
Obrovac, M. N. & Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 7, A93 (2004).
McDowell, M. T., Lee, S. W., Nix, W. D. & Cui, Y. 25th anniversary article: understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 25, 4966–4985 (2013).
McDowell, M. T. et al. In situ TEM of two-phase lithiation of amorphous silicon nanospheres. Nano Lett. 13, 758–764 (2013).
Hulteen, J. C. et al. Nanosphere lithography: size-tunable silver nanoparticle and surface cluster arrays. J. Phys. Chem. B 103, 3854–3863 (1999).
Liu, N. et al. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nature Nanotech. 9, 187–192 (2014).
Liu, Y., Goebl, J. & Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 42, 2610–2653 (2013).
Li, N. et al. Sol-gel coating of inorganic nanostructures with resorcinol-formaldehyde resin. Chem. Commun. 49, 5135–5137 (2013).
Liu, J. et al. Extension of the Stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres. Angew. Chem. Int. Ed. Engl. 50, 5947–5951 (2011).
Zeng, Z., Liang, W.-I., Chu, Y.-H. & Zheng, H. In situ TEM study of the Li–Au reaction in an electrochemical liquid cell. Faraday Discuss. 176, 95–107 (2014).
Massalski, T. B. & Okamoto, H. Binary Alloy Phase Diagrams (ASM International, 1990).
Moon, G. D. et al. Assembled monolayers of hydrophilic particles on water surfaces. ACS Nano 5, 8600–8612 (2011).
Stöber, W., Fink, A. & Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 26, 62–69 (1968).
Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 241, 20–22 (1973).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Battery Materials Research (BMR) Program in the Office of Vehicle Technologies of the US Department of Energy. H.-W.L. was supported by Basic Science Research Program through the National Research Foundation of Korea under Contract No. NRF-2012R1A6A3A03038593.
Author information
Authors and Affiliations
Contributions
K.Y. and Y.C. conceived the idea. K.Y., F.X. and P.-C.H. conducted the film deposition. K.Y. and Z.L. synthesized hollow spheres. K.Y. carried out the electrochemical tests. H.-W.L., Y.L. and Z.L. performed TEM characterizations. K.Y., P.-C.H., J.Z. and Z.L. worked on other characterizations. Y.C. and S.C. supervised the project and participated in the planning of research. K.Y., S.C. and Y.C. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–7, Supplementary Reference. (PDF 4730 kb)
Supplementary Video 1
In-situ lithium melting. Two examples of in situ melting of lithium metal particles encapsuled inside a nanocapsule, after electrochemical deposition. Nanocapsules with a single gold nanoparticle enclosed were used for clarity. (MOV 11831 kb)
Supplementary Video 2
In-situ lithium filling into nanocapsules. Lithium deposition inside nanocapsules is revealed by in situ transmission electron microscopy. The set-up was biased at a constant potential. Because the resistance of the cell decreased as lithium was gradually injected into the carbon structure, the lithiation/lithium-plating speed accelerated during the test. The playback speed changed at the point when lithium started to plate, to compensate the actual speed change. (MOV 12509 kb)
Supplementary Video 3
In-situ lithium cycling in nanocapsules. In situ observation of lithium cycling inside a nanocapsule. Carbon shells with single gold nanoparticles were used here for clarity, although tiny gold nanoparticles still exist on the inner wall of the carbon shell, which altered the nucleation of lithium at the first cycle. The gold nanoparticle was able to dissolve in lithium metal completely due to e-beam heating. Precipitation of gold was confined within the identical carbon shell, although the exact position may be random. (MOV 21885 kb)
Rights and permissions
About this article
Cite this article
Yan, K., Lu, Z., Lee, HW. et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat Energy 1, 16010 (2016). https://doi.org/10.1038/nenergy.2016.10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.10
This article is cited by
-
Enabling an Inorganic-Rich Interface via Cationic Surfactant for High-Performance Lithium Metal Batteries
Nano-Micro Letters (2024)
-
A free-standing lithium phosphorus oxynitride thin film electrolyte promotes uniformly dense lithium metal deposition with no external pressure
Nature Nanotechnology (2023)
-
Gradient design of imprinted anode for stable Zn-ion batteries
Nature Communications (2023)
-
Making the deposition surface lithiophobic
Nature Energy (2023)
-
Monolithically-stacked thin-film solid-state batteries
Communications Chemistry (2023)