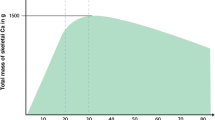
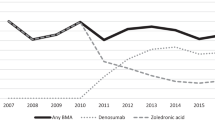
Abstract
Prostate cancer is the second most common cancer diagnosed in men worldwide and, although advances in treatment options have extended the overall survival of these patients, bone health issues remain a challenge throughout the continuum of care. Patients with prostate cancer are at high risk of skeletal complications from bone metastases and bone loss induced by cancer treatments, such as androgen-deprivation therapy. The preservation of skeletal health might require the cooperation of urologists, oncologists, pain specialists, and other physicians specializing in the treatment of prostate cancer. Complications resulting from bone loss and bone metastases can result in increased risk of fracture and death. Implementation of a multidisciplinary approach for the management of bone health can, therefore, provide clinically meaningful benefits to patients with skeletal complications. The early diagnosis and treatment of bone loss and bone metastases with bisphosphonates are critical for the maintenance of skeletal wellness and prevention of bone complications in patients with prostate cancer.
Key Points
-
Bone health issues remain a challenge throughout the continuum of care in patients with prostate cancer
-
The early diagnosis and treatment with bisphosphonates of bone loss and bone metastases in patients with prostate cancer are critical to maintain skeletal wellness and prevent bone complications
-
Although the use of biomarkers of bone metabolism, imaging techniques, and adjuvant therapies for the diagnosis and treatment of skeletal complications are being developed, risk-assessment tools are needed for the identification of patients with prostate cancer who are at an increased risk of skeletal disease
-
The cooperation of urologists, medical oncologists, radiation oncologists, and other specialists to ensure that bone health is monitored and treated throughout the course of disease progression can optimize outcomes
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Parkin DM et al. (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108
Tannock IF et al. (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351: 1502–1512
Petrylak DP et al. (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351: 1513–1520
Preston DM et al. (2002) Androgen deprivation in men with prostate cancer is associated with an increased rate of bone loss. Prostate Cancer Prostatic Dis 5: 304–310
Shahinian VB et al. (2005) Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352: 154–164
Smith MR et al. (2005) Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol 23: 7897–7903
Coleman RE (2004) Bisphosphonates: clinical experience. Oncologist 9 (Suppl 4): 14–27
Saad F et al. (2004) Long-term efficacy and safety of zoledronic acid in men with advanced prostate cancer and bone metastases. Presented at XIXth Congress of the European Association of Urology March 24–27, Vienna, Austria
Lipton A (2004) Pathophysiology of bone metastases: how this knowledge may lead to therapeutic intervention. J Support Oncol 2: 205–213
Weinfurt KP et al. (2005) The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 16: 579–584
Oefelein MG et al. (2002) Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 168: 1005–1007
Saad F et al. (2006) Pathologic fractures and bone markers are predictors of clinical outcome in patients with prostate cancer and bone metastases. Presented at 2006 Prostate Cancer Symposium February 24–26, San Francisco, CA, USA
Noguchi M et al. (2003) Percentage of the positive area of bone metastasis is an independent predictor of disease death in advanced prostate cancer. Br J Cancer 88: 195–201
Pummer K (2006) Therapy of hormone refractory prostate cancer. Presented at 21st Annual Congress of the European Association of Urology April 5–8, Paris, France
Polascik TJ et al. (2005) Open-label trial evaluating the safety and efficacy of zoledronic acid in preventing bone loss in patients with hormone-sensitive prostate cancer and bone metastases. Urology 66: 1054–1059
Sternberg CN et al. (2006) The medical management of prostate cancer: a multidisciplinary team approach. BJU Int 99: 22–27
(1999) The Royal College of Radiologists' Clinical Oncology Information Network. British Association of Urological Surgeons. Guidelines on the management of prostate cancer. Clin Oncol (R Coll Radiol) 11: S53–S88
Aus G et al. (2005) EAU guidelines on prostate cancer. Eur Urol 48: 546–551
Diamond TH et al. (2004) Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer 100: 892–899
Dearnaley DP et al. (2003) A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 95: 1300–1311
Saad F et al. (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94: 1458–1468
Smith JA Jr (1989) Palliation of painful bone metastases from prostate cancer using sodium etidronate: results of a randomized, prospective, double-blind, placebo-controlled study. J Urol 141: 85–87
Ernst DS et al. (2003) Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol 21: 3335–3342
Heidenreich A et al. (2005) Ibandronate in the management of painful osseous metastases due to hormone refractory prostate cancer. Presented at 2005 Prostate Cancer Symposium February 17–19, Orlando, FL, USA
Lipton A et al. (2002) The new bisphosphonate, Zometa (zoledronic acid), decreases skeletal complications in both osteolytic and osteoblastic lesions: a comparison to pamidronate. Cancer Invest 20 (Suppl 2): 45–54
Small EJ et al. (2003) Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol 21: 4277–4284
Major PP et al. (2000) Oral bisphosphonates: a review of clinical use in patients with bone metastases. Cancer 88: 6–14
Greenspan SL et al. (2005) Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 90: 6410–6417
Maillefert JF et al. (1999) Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol 161: 1219–1222
Smith MR et al. (2006) Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol 175: 136–139
Saad F et al. (2006) Natural history and treatment of bone complications in prostate cancer. Eur Urol 49: 429–440
Higano C (2006) Androgen deprivation therapy: monitoring and managing the complications. Hematol Oncol Clin North Am 20: 909–923
Kaneko Y et al. (2006) Intermittent androgen deprivation therapy may prolong the duration of androgen dependence of well-differentiated prostate cancer. Hinyokika Kiyo 52: 259–264
Sharifi N et al. (2005) Androgen deprivation therapy for prostate cancer. JAMA 294: 238–244
Nelson JB et al. (2006) Once weekly oral alendronate prevents bone loss in men on androgen deprivation therapy for prostate cancer. Presented at 2006 Prostate Cancer Symposium February 24–26, San Francisco, CA, USA
Smith MR et al. (2004) Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab 89: 3841–3846
Smith MR et al. (2003) Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 169: 2008–2012
Casey R et al. (2006) Zoledronic acid reduces bone loss in men with prostate cancer undergoing androgen deprivation therapy. Presented at 2006 Prostate Cancer Symposium February 24–26, San Francisco, CA, USA
Padalecki SS et al. (2003) Androgen deprivation causes bone loss and increased prostate cancer metastases to bone: Prevention by zoledronic acid. Presented at 24th Annual Meeting of the American Society of Bone and Mineral Research September 20–24, San Antonio, TX, USA
Mystakidou K et al. (2005) Randomized, open label, prospective study on the effect of zoledronic acid on the prevention of bone metastases in patients with recurrent solid tumors that did not present with bone metastases at baseline. Med Oncol 22: 195–201
Saad F et al. (2004) Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96: 879–882
Serafini AN (2001) Therapy of metastatic bone pain. J Nucl Med 42: 895–906
Rosen LS et al. (2004) Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer 100: 2613–2621
Rosen LS et al. (2004) Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 100: 36–43
Rosen LS et al. (2003) Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 21: 3150–3157
Efstathiou E et al. (2005) Combination of docetaxel, estramustine phosphate, and zoledronic acid in androgen-independent metastatic prostate cancer: efficacy, safety, and clinical benefit assessment. Urology 65: 126–130
Fulfaro F et al. (2005) The use of zoledronic acid in patients with bone metastases from prostate carcinoma: effect on analgesic response and bone metabolism biomarkers. J Chemother 17: 555–559
Ripamonti C et al. (2006) Pain on movement and pain at rest decrease after zoledronic acid infusion in patients with bone metastases due to breast or prostate cancer. J Clin Oncol 24 (Suppl): 694s
Stanculeanu DL et al. (2006) Zoledronic acid treatment in osteocondensant bone metastasis prostate cancer patients. Presented at 2006 Prostate Cancer Symposium February 24–26, San Francisco, CA, USA
Wiktor-Jedrzejczak W (2005) Reduction of pain as the primary determinant of improved quality of life of cancer patients receiving zoledronic acid (Zol) for bone involvement. J Clin Oncol 23 (Suppl): 734s
Smith MR et al. (2005) Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol 23: 2918–2925
Saad F et al. (2006) Predictors of clinical outcome in patients with bone metastases from prostate cancer. J Clin Oncol 24 (Suppl): 230s
Eastham J et al. (2005) Effect of zoledronic acid on bone pain and skeletal morbidity in patients with advanced prostate cancer; analysis by baseline pain. J Clin Oncol 23 (Suppl): 393s
Murray R et al. (2004) Clinical benefit of zoledronic acid for the prevention of multiple skeletal complications in patients with prostate cancer, based on history of skeletal complications. Presented at 29th European Society for Medical Oncology Congress October 29–November 2 Vienna, Austria
Saad F et al. (2006) Zoledronic acid reduces skeletal morbidity regardless of previous skeletal events in men with prostate cancer and bone metastases. Presented at 2006 European Association of Urology 21st Annual Congress April 5–8, Paris, France
McKiernan JM et al. (2004) Impact of skeletal complications on total medical care costs in prostate cancer patients with bone metastases. J Clin Oncol 22 (Suppl): 531
Botteman M et al. (2006) Cost-effectiveness of zoledronic acid vs. pamidronate in the management of hormone refractory prostate cancer (HRPC) patients with bone metastases. J Clin Oncol 24 (Suppl): 649s
Botteman MF et al. (2005) Cost effectiveness of intravenous (IV) zoledronic acid versus other IV bisphosphonates for the prevention of bone complications in breast cancer patients with bone metastases: a Markov model from the UK perspective. J Clin Oncol 23 (Suppl): 58s
Saad F et al. (2003) Zoledronic acid is well tolerated for up to 24 months and significantly reduces skeletal complications in patients with advanced prostate cancer metastatic to bone. Presented at American Urological Association Annual Meeting April 26–May 1, Chicago, IL, USA
Farrugia MC et al. (2006) Osteonecrosis of the mandible or maxilla associated with the use of new generation bisphosphonates. Laryngoscope 116: 115–120
Ficarra G et al. (2005) Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J Clin Periodontol 32: 1123–1128
Marx RE et al. (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63: 1567–1575
Melo MD and Obeid G (2005) Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. J Am Dent Assoc 136: 1675–1681
Mignogna MD et al. (2006) Case 2. Osteonecrosis of the jaws associated with bisphosphonate therapy. J Clin Oncol 24: 1475–1477
Olson KB et al. (2005) Osteonecrosis of jaw in patient with hormone-refractory prostate cancer treated with zoledronic acid. Urology 66: 658
Pastor-Zuazaga D et al. (2006) Osteonecrosis of the jaws and bisphosphonates. Report of three cases. Med Oral Patol Oral Cir Bucal 11: E76–E79
Ruggiero SL et al. (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62: 527–534
Woo SB et al. (2006) Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaw. Ann Intern Med 144: 753–761
Hoff AO et al. (2006) Osteonecrosis of the jaw in patients receiving intravenous bisphosphonate therapy. Presented at: 42nd Annual Meeting of the American Society of Clinical Oncology June 2–6, Atlanta, GA, USA
Ruggiero S et al. (2006) Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract 2: 7–14
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27: 165–176
Hillner BE et al. (2003) American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21: 4042–4057
Acknowledgements
We acknowledge the assistance of Carol Sledz in the editorial preparation of this manuscript before submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
F Saad has received funding for clinical research from Novartis Pharmaceuticals Corporation and Sanofi Aventis and has participated in such advisory boards.
CN Sternberg has participated in Novartis Pharmaceuticals Corporation and Sanofi Aventis advisory boards.
Rights and permissions
About this article
Cite this article
Saad, F., Sternberg, C. Multidisciplinary management of bone complications in prostate cancer and optimizing outcomes of bisphosphonate therapy. Nat Rev Urol 4 (Suppl 1), S3–S13 (2007). https://doi.org/10.1038/ncpuro0727
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0727
This article is cited by
-
Is 153Samarium-ethylene-diamine-tetramethyl-phosphonate (EDTMP) bone uptake influenced by bisphosphonates in patients with castration-resistant prostate cancer?
World Journal of Urology (2012)
-
Bone metastasis in prostate cancer: emerging therapeutic strategies
Nature Reviews Clinical Oncology (2011)