Abstract
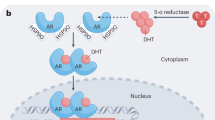
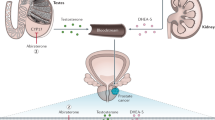
The prevalence of prostate cancer emphasizes the need for improved therapeutic options, particularly for metastatic disease. Current treatment includes medical or surgical castration, which initially induces apoptosis of prostate cancer cells, but ultimately an androgen-independent subpopulation emerges. In addition to a transient therapeutic effect, androgen-deprivation therapy (ADT) can initiate biochemical events that may contribute to the development of and progression to an androgen-independent state. This transition involves multiple signal transduction pathways that are accompanied by many biochemical changes resulting from ADT. These molecular events themselves are therapeutic targets and serve as a rationale for adjunctive treatment at the time of ADT.
Key Points
-
Although not completely understood, the progression of prostate cancer to an androgen-independent state probably involves multiple biochemical pathways
-
Therapeutic androgen ablation is likely to be an initial factor driving this biochemical cascade of events
-
Novel biologic agents are now available enabling modification of some of the pathways involved in the development of androgen-independent prostate cancer
-
Current clinical data shows some efficacy for biologic agents when used with chemotherapy in the setting of androgen-independent disease
-
Future trials should also test biologic agents at the time of androgen ablation to attempt to maximize the initial apoptotic response and delay the onset of androgen independence
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal A et al. (2006) Cancer statistics, 2006. CA Cancer J Clin 56: 106–130
Smaletz O et al. (2002) Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol 20: 3972–3982
Sharifi N and Farrar WL (2006) Androgen receptor as a therapeutic target for androgen independent prostate cancer. Am J Ther 13: 166–170
Feldman BJ and Feldman D (2001) The development of androgen-independent prostate cancer. Nat Rev Cancer 1: 34–45
So A et al. (2005) Mechanisms of the development of androgen independence in prostate cancer. World J Urol 23: 1–9
Wu RC et al. (2005) Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocr Rev 26: 393–399
Culig Z et al. (2004) Expression and function of androgen receptor coactivators in prostate cancer. J Steroid Biochem Mol Biol 92: 265–271
Gioeli D et al. (1999) Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res 59: 279–284
Lorenzo GD et al. (2003) Involvement of growth factor receptors of the epidermal growth factor receptor family in prostate cancer development and progression to androgen independence. Clin Prostate Cancer 2: 50–57
Lara PN Jr et al. (2004) Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California Cancer Consortium Screening and Phase II Trial. Cancer 100: 2125–2131
Ziada A et al. (2004) The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate 60: 332–337
van der Poel HG (2004) Smart drugs in prostate cancer. Eur Urol. 45: 1–17
Smith MR and Nelson JB (2005) Future therapies in hormone-refractory prostate cancer. Urology 65: 9–16; discussion 17
Canil CM et al. (2005) Randomized phase II study of two doses of gefitinib in hormone-refractory prostate cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol 23: 455–460
Krueckl SL et al. (2004) Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res 64: 8620–8629
Miyake H et al. (2000) Castration-induced up-regulation of insulin-like growth factor binding protein-5 potentiates insulin-like growth factor-I activity and accelerates progression to androgen independence in prostate cancer models. Cancer Res 60: 3058–3064
Kiyama S et al. (2003) Castration-induced increases in insulin-like growth factor-binding protein 2 promotes proliferation of androgen-independent human prostate LNCaP tumors. Cancer Res 63: 3575–3584
Miyata Y et al. (2004) Expression of insulin-like growth factor binding protein-3 before and after neoadjuvant hormonal therapy in human prostate cancer tissues: correlation with histopathologic effects and biochemical recurrence. Urology 63: 1184–1190
Papandreou CN et al. (1998) Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat Med 4: 50–57
Lee LF et al. (2001) Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol 21: 8385–8397
Amorino GP and Parsons SJ (2004) Neuroendocrine cells in prostate cancer. Crit Rev Eukaryot Gene Expr 14: 287–300
Song L et al. (2006) Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res 66: 5542–5548
Yang JC et al. (2005) Src kinase inhibition of neuropeptide-induced androgen-independent prostate cancer. Proc Amer Assoc Cancer Res 46: 3180
Nam S et al. (2005) Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res 65: 9185–9189
McDonnell TJ et al. (1992) Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 52: 6940–6944
Colombel M et al. (1993) Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am J Pathol 143: 390–400
Shi Y et al. (2006) Role of coordinated molecular alterations in the development of androgen-independent prostate cancer: an in vitro model that corroborates clinical observations. BJU Int 97: 170–178
Miyake H et al. (2001) Novel therapeutic strategy for advanced prostate cancer using antisense oligodeoxynucleotides targeting anti-apoptotic genes upregulated after androgen withdrawal to delay androgen-independent progression and enhance chemosensitivity. Int J Urol 8: 337–349
Tolcher AW et al. (2005) A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res 11: 3854–3861
Gleave M et al. (2005) Beyond simple castration: targeting the molecular basis of treatment resistance in advanced prostate cancer. Cancer Chemother Pharmacol 56 (Suppl 1): 47–57
July LV et al. (2002) Clusterin expression is significantly enhanced in prostate cancer cells following androgen withdrawal therapy. Prostate 50: 179–188
Miyake H et al. (2005) Antisense oligodeoxynucleotide therapy targeting clusterin gene for prostate cancer: Vancouver experience from discovery to clinic. Int J Urol 12: 785–794
Chi KN et al. (2005) A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2′-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst 97: 1287–1296
Baretton GB et al. (1999) Proliferation- and apoptosis-associated factors in advanced prostatic carcinomas before and after androgen deprivation therapy: prognostic significance of p21/WAF1/CIP1 expression. Br J Cancer 80: 546–555
Wikstrom P et al. (1999) Early castration-induced upregulation of transforming growth factor beta1 and its receptors is associated with tumor cell apoptosis and a major decline in serum prostate-specific antigen in prostate cancer patients. Prostate 38: 268–277
Chevalier S et al. (2002) Vascular endothelial growth factor and signaling in the prostate: more than angiogenesis. Mol Cell Endocrinol 189: 169–179
George DJ et al. (2001) Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res 7: 1932–1936
Lara PN Jr et al. (2004) Angiogenesis-targeted therapies in prostate cancer. Clin Prostate Cancer 3: 165–173
Picus J et al. (2003) The use of bevacizumab (B) with docetaxel (D) and estramustine (E) in hormone refractory prostate cancer (HRPC): Initial results of CALGB 90006. Proc Am Soc Clin Oncol 22: 393
Schlaepfer DD and Hunter T (1998) Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol 8: 151–157
Danen EH and Yamada KM (2001) Fibronectin, integrins, and growth control. J Cell Physiol 189: 1–13
Stewart DA et al. (2004) Changes in extracellular matrix (ECM) and ECM-associated proteins in the metastatic progression of prostate cancer. Reprod Biol Endocrinol 2: 2
Stubbs AP et al. (1999) Differentially expressed genes in hormone refractory prostate cancer: association with chromosomal regions involved with genetic aberrations. Am J Pathol 154: 1335–1343
Legrier ME et al. (2004) Mucinous differentiation features associated with hormonal escape in a human prostate cancer xenograft. Br J Cancer 90: 720–727
North SA et al. (2006) A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J Urol 176: 91–95
Kousidou O et al. (2006) Effects of the natural isoflavonoid genistein on growth, signaling pathways and gene expression of matrix macromolecules by breast cancer cells. Mini Rev Med Chem 6: 331–337
Papandreou CN and Logothetis CJ (2004) Bortezomib as a potential treatment for prostate cancer. Cancer Res 64: 5036–5043
Orlowski RZ and Baldwin AS Jr (2002) NF-kappaB as a therapeutic target in cancer. Trends Mol Med 8: 385–389
Price N and Dreicer R (2004) Phase I/II trial of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin Prostate Cancer 3: 141–143
Papandreou CN et al. (2004) Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol 22: 2108–2121
Petrylak DP et al. (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351: 1513–1520
Tannock IF et al. (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351: 1502–1512
Chung LW et al. (2005) Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 173: 10–20
Gregory CW et al. (1998) Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res 58: 5718–5724
Hong H et al. (2004) Aberrant expression of CARM1, a transcriptional coactivator of androgen receptor, in the development of prostate carcinoma and androgen-independent status. Cancer 101: 83–89
Debes JD et al. (2002) p300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6. Cancer Res 62: 5632–5636
Halkidou K et al. (2003) Expression of Tip60, an androgen receptor coactivator, and its role in prostate cancer development. Oncogene 22: 2466–2477
Culig Z et al. (1994) Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 54: 5474–5478
Dorkin TJ et al. (1999) aFGF immunoreactivity in prostate cancer and its co-localization with bFGF and FGF8. J Pathol 189: 564–569
Sirotnak FM et al. (2004) Microarray analysis of prostate cancer progression to reduced androgen dependence: studies in unique models contrasts early and late molecular events. Mol Carcinog 41: 150–163
Bubendorf L et al. (1999) Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Inst 91: 1758–1764
Stewart RJ et al. (2001) Vascular endothelial growth factor expression and tumor angiogenesis are regulated by androgens in hormone responsive human prostate carcinoma: evidence for androgen dependent destabilization of vascular endothelial growth factor transcripts. J Urol 165: 688–693
Aslan G et al. (2005) Vascular endothelial growth factor expression in untreated and androgen-deprived patients with prostate cancer. Pathol Res Pract 201: 593–598
Zellweger T et al. (2005) Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer 113: 619–628
Naimi B et al. (2002) Down-regulation of (IIIb) and (IIIc) isoforms of fibroblast growth factor receptor 2 (FGFR2) is associated with malignant progression in human prostate. Prostate 52: 245–252
Dizeyi N et al. (2005) Expression of serotonin receptors 2B and 4 in human prostate cancer tissue and effects of their antagonists on prostate cancer cell lines. Eur Urol 47: 895–900
Jongsma J et al. (2000) Androgen deprivation of the PC-310 [correction of prohormone convertase-310] human prostate cancer model system induces neuroendocrine differentiation. Cancer Res 60: 741–748
Salido M et al. (2002) Neuropeptides bombesin and calcitonin inhibit apoptosis-related elemental changes in prostate carcinoma cell lines. Cancer 94: 368–377
Takahashi W et al. (2003) Regulatory effect of castration on endothelins, their receptors and endothelin-converting enzyme in rat seminal vesicle. BJU Int 92: 803–809
Yashi M et al. (2003) Elevated serum progastrin-releasing peptide (31-98) level is a predictor of short response duration after hormonal therapy in metastatic prostate cancer. Prostate 56: 305–312
Levine L et al. (2003) Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res 63: 3495–3502
Yuan TC et al. (2006) Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer 13: 151–167
Vashchenko N and Abrahamsson PA (2005) Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol 47: 147–155
Gutierrez-Canas I et al. (2005) Vasoactive intestinal peptide induces neuroendocrine differentiation in the LNCaP prostate cancer cell line through PKA, ERK, and PI3K. Prostate 63: 44–55
Sugihara A et al. (1998) Expression of cytokines enhancing the osteoclast activity, and parathyroid hormone-related protein in prostatic cancers before and after endocrine therapy: an immunohistochemical study. Oncol Rep 5: 1389–1394
Wise GJ et al. (2000) Cytokine variations in patients with hormone treated prostate cancer. J Urol 164: 722–725
Lee LF et al. (2004) Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene 23: 2197–2205
Huerta-Yepez S et al. (2006) Involvement of the TNF-alpha autocrine-paracrine loop, via NF-kappaB and YY1, in the regulation of tumor cell resistance to Fas-induced apoptosis. Clin Immunol 120: 297–309
Watson RW and Fitzpatrick JM (2005) Targeting apoptosis in prostate cancer: focus on caspases and inhibitors of apoptosis proteins. BJU Int 96 (Suppl 2): 30–34
Koivisto PA and Rantala I (1999) Amplification of the androgen receptor gene is associated with P53 mutation in hormone-refractory recurrent prostate cancer. J Pathol 187: 237–241
Pflug BR et al. (1999) Caveolin expression is decreased following androgen deprivation in human prostate cancer cell lines. Prostate 40: 269–273
Persad S and Dedhar S (2003) The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev 22: 375–384
Miyamoto H et al. (2005) Inhibition of the Akt, cyclooxygenase-2, and matrix metalloproteinase-9 pathways in combination with androgen deprivation therapy: potential therapeutic approaches for prostate cancer. Mol Carcinog 44: 1–10
Kiviniemi J et al. (2004) Altered expression of syndecan-1 in prostate cancer. Apmis 112: 89–97
Lokeshwar BL (1999) MMP inhibition in prostate cancer. Ann N Y Acad Sci 878: 271–289
Bratland A et al. (2003) The metalloproteinase inhibitor TIMP-2 is down-regulated by androgens in LNCaP prostate carcinoma cells. Clin Exp Metastasis 20: 541–547
Nishiyama T et al. (2004) The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res 10: 7121–7126
Lei Q et al. (2006) NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell 9: 367–378
Majumder PK and Sellers WR (2005) Akt-regulated pathways in prostate cancer. Oncogene 24: 7465–7474
Edwards J et al. (2004) The role of c-Jun and c-Fos expression in androgen-independent prostate cancer. J Pathol 204: 153–158
Aprikian AG et al. (1996) Bombesin specifically induces intracellular calcium mobilization via gastrin-releasing peptide receptors in human prostate cancer cells. J Mol Endocrinol 16: 297–306
Sumitomo M et al. (2001) Neutral endopeptidase inhibits neuropeptide-mediated transactivation of the insulin-like growth factor receptor-Akt cell survival pathway. Cancer Res 61: 3294–3298
Devi GR et al. (2005) In vivo bioavailability and pharmacokinetics of a c-MYC antisense phosphorodiamidate morpholino oligomer, AVI-4126, in solid tumors. Clin Cancer Res 11: 3930–3938
Smith MR et al. (2006) Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol 24: 2723–2728
Pruthi RS et al. (2003) Cyclooxygenase-2 as a potential target in the prevention and treatment of genitourinary tumors: a review. J Urol 169: 2352–2359
Ling MT et al. (2004) Id-1 expression induces androgen-independent prostate cancer cell growth through activation of epidermal growth factor receptor (EGF-R). Carcinogenesis 25: 517–525
Ettinger SL et al. (2004) Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res 64: 2212–2221
Sasaki T et al. (2005) Changes in chromogranin a serum levels during endocrine therapy in metastatic prostate cancer patients. Eur Urol 48: 224-229; discussion 229–230
Imasato Y et al. (2000) PSP94 expression after androgen deprivation therapy: a comparative study with prostate specific antigen in benign prostate and prostate cancer. J Urol 164: 1819–1824
Rittmaster RS et al. (1999) The utility of tissue transglutaminase as a marker of apoptosis during treatment and progression of prostate cancer. J Urol 162: 2165–2169
Acknowledgements
Research supported in part by NIH grant KO8 DK60748-01 and Department of Defense grant PC040161.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nelson, E., Cambio, A., Yang, J. et al. Biologic agents as adjunctive therapy for prostate cancer: a rationale for use with androgen deprivation. Nat Rev Urol 4, 82–94 (2007). https://doi.org/10.1038/ncpuro0700
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0700
This article is cited by
-
Identification of molecular targets in urologic oncology
World Journal of Urology (2009)
-
Inhibition of Akt pathways in the treatment of prostate cancer
Prostate Cancer and Prostatic Diseases (2007)