Abstract
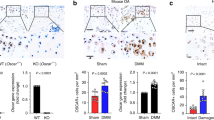
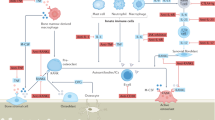
Chronic inflammation and bone loss are closely linked pathophysiologic events. The most typical example of inflammatory bone loss is seen in patients with rheumatoid arthritis who develop systemic osteopenia as well as local breakdown of bone in the direct vicinity of inflamed joints. Understanding the mechanisms of arthritic bone degradation is crucial for designing therapies that can specifically protect joints from structural damage. Since osteoclast differentiation and activity are key events in arthritic bone damage, the signals that trigger osteoclastogenesis are potential therapeutic targets. Receptor activator of nuclear factor-κB (RANK) is activated by its ligand, RANKL, an essential molecule for osteoclast development: in the absence of RANKL or RANK, osteoclast differentiation from monocyte precursors does not occur. RANKL is expressed on T cells and fibroblasts within the synovial inflammatory tissue of patients with RA and its expression is regulated by proinflammatory cytokines. In animal models of arthritis, blockade of RANKL–RANK interactions, or a genetic absence of RANKL or RANK, protects against joint damage despite the presence of joint inflammation. Therefore, inhibition of RANKL is regarded as a promising future strategy for inhibiting inflammatory bone loss in patients with chronic inflammatory arthritis.
Key Points
-
Chronic synovial inflammation triggers local as well as generalized bone loss
-
The link between chronic inflammation and skeletal breakdown lies in the potential of synovial inflammatory tissue to induce the formation of osteoclasts
-
Receptor activator of NFκB ligand (RANKL) is an essential molecule for osteoclastogenesis
-
Therapeutic inhibition of RANKL is effective for preventing local bone erosion in animal models of arthritis
-
RANKL inhibition is therefore considered to be a promising therapeutic tool for protecting bone against arthritic damage
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karsenty G (2003) The complexities of skeletal biology. Nature 423: 316–318
Goldring S (2003) Osteoporosis and rheumatic diseases. In Primer of the metabolic bone diseases and disorders of mineral metabolism. Edn 5 (Ed. Favus MJ) New York: Lippincott Williams & Wilkins
Woolf AD (1991) Osteoporosis in rheumatoid arthritis—the clinical viewpoint. Br J Rheumatol 30: 82–84
Spector TD et al. (1993) Risk of vertebral fracture in women with rheumatoid arthritis. Br Med J 306: 558
Gough AK et al. (1994) Generalized bone loss in patients with early rheumatoid arthritis. Lancet 344: 23–27
Kvien TK et al. (2000) Data driven attempt to create a clinical algorithm for identification of women with rheumatoid arthritis at high risk of osteoporosis. Ann Rheum Dis 59: 805–811
Cooper C et al. (1995) Rheumatoid arthritis, corticosteroid therapy and hip fracture. Ann Rheum Dis 54: 49–52
Schett G et al. (2003) Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum 48: 2042–2051
Weichselbaum A (1878) Die feineren veränderungen des gelenkknorpels bei fungöser synovitis und karies der gelenkenden. Archiv Pathol Anat Physiol Klin Med 73: 461–475
Arnett FC et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31: 315–324
Sharp JT et al. (1985) How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum 28: 1326–1335
Scott DL et al. (2000) The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford) 39: 122–132
Pincus T (1988) Rheumatoid arthritis: disappointing long-term outcomes despite successful short-term clinical trials. J Clin Epidemiol 41: 1037–1041
Welsing PM et al. (2001) The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum 44: 2009–2017
Bromley M and Woolley DE (1984) Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum 27: 968–975
Gravallese EM et al. (1998) Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 152: 943–951
Redlich K et al. (2002) Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest 110: 1419–1427
Pettit AR et al. (2001) TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol 159: 1689–1699
Li P et al. (2004) Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11b-high osteoclast precursors in tumor necrosis factor α-transgenic mice. Arthritis Rheum 50: 265–276
Grigoriadis AE et al. (1994) c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266: 443–448
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289: 1504–1508
Lacey DL et al. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176
Yasuda H et al. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/ RANKL. Proc Natl Acad Sci USA 95: 3597–3602
Kong YY et al. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397: 315–323
Lomaga MA et al. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13: 1015–1024
Wada T et al. (2005) The molecular scaffold Gab2 is a crucial component of RANK signaling and osteoclastogenesis. Nat Med 11: 394–399
Iotsova V et al. (1997) Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med 3: 1285–1289
Ruocco MG et al. (2005) IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med 201: 1677–1687
Lam J et al. (2000) TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 106: 1481–1488
Wei S et al. (2005) IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest 115: 282–290
Gravallese EM et al. (2000) Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 43: 250–258
Shigeyama Y et al. (2000) Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum 43: 2523–2530
Ritchlin CT et al. (2003) Mechanisms of TNF-α- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest 111: 821–831
Ziolkowska M et al. (2002) High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis Rheum 46: 1744–1753
Lubberts E et al. (2002) Increase in expression of receptor activator of nuclear factor κB at sites of bone erosion correlates with progression of inflammation in evolving collagen-induced arthritis. Arthritis Rheum 46: 3055–3064
Schett G et al. (2000) Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum 43: 2501–2512
Schett G et al. (2005) Analysis of the kinetics of osteoclastogenesis in arthritic rats. Arthritis Rheum 52: 3192–3201
Van der Heijde DM (1995) Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol 34: 74–78
Simonet WS et al. (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319
Kong YY et al. (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304–309
Redlich, K et al. (2002) Tumor necrosis factor α-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum 46: 785–792
Romas E et al. (2002) Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol 161: 1419–1427
Lubberts E et al. (2003) IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-κB ligand/osteoprotegerin balance. J Immunol 170: 2655–2662
Herrak P et al. (2004) Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor-mediated arthritis. Arthritis Rheum 50: 2327–2337
Sims NA et al. (2004) Targeting osteoclasts with zoledronic acid prevents bone destruction in collagen-induced arthritis. Arthritis Rheum 50: 2338–2346
Goldring S and Gravallese EM (2004) Bisphosphonates: environmental protection for the joint? Arthritis Rheum 46: 2044–2047
Bekker PJ et al. (2001) The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 16: 348–360
Bekker PJ et al. (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19: 1059–1066
Schett G et al. (2004) Soluble RANKL and risk of nontraumatic fracture. JAMA 291: 1108–1113
Zwerina J et al. (2004) Single and combined inhibition of tumor necrosis factor, IL-1 and RANKL-pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum 50: 277–290
Komuro H et al. (2001) The osteoprotegerin/receptor activator of nuclear factor κB/receptor activator of nuclear factor κB ligand system in cartilage. Arthritis Rheum 44: 2768–2776
Kiechl S et al. (2004) Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 109: 2175–2180
Redlich K et al. (2004) Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. Am J Pathol 164: 543–555
Acknowledgements
G Schett receives funding support from START Program of the Austrian Science Fund (FWF) of the Austrian Federal Ministry for Education, Science and Culture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schett, G., Hayer, S., Zwerina, J. et al. Mechanisms of Disease: the link between RANKL and arthritic bone disease. Nat Rev Rheumatol 1, 47–54 (2005). https://doi.org/10.1038/ncprheum0036
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0036
This article is cited by
-
Comparative study of the synovial levels of RANKL and OPG in rheumatoid arthritis, spondyloarthritis and osteoarthritis
Advances in Rheumatology (2023)
-
Chronic arthritides and bone structure: focus on rheumatoid arthritis—an update
Aging Clinical and Experimental Research (2023)
-
Effect of denosumab switched from bisphosphonates in preventing joint destruction in postmenopausal rheumatoid arthritis patients with anti-cyclic citrullinated peptide antibodies
Journal of Orthopaedic Surgery and Research (2021)
-
Effect of resolvin D5 on T cell differentiation and osteoclastogenesis analyzed by lipid mediator profiling in the experimental arthritis
Scientific Reports (2021)
-
Effects of one-year tofacitinib therapy on bone metabolism in rheumatoid arthritis
Osteoporosis International (2021)