Abstract
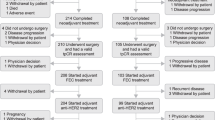
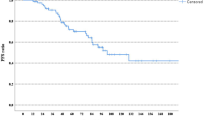
Trastuzumab has an established role for the treatment of HER2-positive early-stage breast cancer because of the success of this agent in the adjuvant setting. Several key questions about the value of trastuzumab for the treatment of breast cancer, however, still need to be answered. Various differences in patient characteristics and treatment regimens were present in the randomized trials discussed in this Review; therefore, the details of trastuzumab use need clarification. For example, the optimum timing, the ideal administration schedule, and the appropriate length of treatment are not known. Cardiotoxicity is major concern even though the results of all randomized trials have shown that the degree of cardiotoxicity with trastuzumab is acceptable—the incidence of cardiac damage caused by trastuzumab ranged from 0.4% to 4.1% in the different trials (cumulative incidence of congestive heart failure, New York Heart Association class 3–4). Current data do not support the use of trastuzumab for more than 1 year. The analysis of 2-year treatment with trastuzumab is expected to be available in 2009.
Key Points
-
Adjuvant trastuzumab has an established role in daily clinical practice in patients with HER2-positive breast cancer
-
Cardiotoxicity of trastuzumab is major concern, even though the results of all randomized trials have shown that the degree of cardiac toxic effects produced by trastuzumab is acceptable
-
The incidence of cardiac damage due to trastuzumab ranged from 0.4% to 4.1% in the different randomized trials (cumulative incidence of congestive hearth failure, New York Heart Association class 3–4)
-
Several differences exist in the design and outcomes of the randomized trials of adjuvant trastuzumab; as a result, the optimum timing, schedule and length of treatment with trastuzumab is not known
-
Currently data do not support use of trastuzumab for more than 1 year
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Slamon DJ et al. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182
Slamon DJ et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792
Marty M et al. (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23: 4265–4274
Pegram MD et al. (2004) Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst 19: 759–769
Romond EH et al. (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 20: 1673–1684
Piccart-Gebhart MJ et al. for the Herceptin Adjuvant (HERA) Trial Study Team (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 20: 1659–1672
Slamon D et al. (2005) Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC → T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC → TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treat 94 (Suppl 1): S1
Joensuu H et al. for the FinHer Study Investigators (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 23: 809–820
Spielmann M et al. (2007) 3-year follow-up of trastuzumab following adjuvant chemotherapy in node positive HER2-positive breast cancer patients: results of the PACS-04 trial. Breast Cancer Res Treat 106 (Suppl 1): S72
Gasparini G et al. (2006) Randomized phase II trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat 19: 9306–9309
Esteva FJ et al. (2002) Phase II study of weekly docetaxel and trastuzumab for patients with HER-2 overexpressing metastatic breast cancer. J Clin Oncol 20: 1800–1808
Burstein HJ et al. (2003) Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors and cardiac surveillance algorithm. J Clin Oncol 21: 2889–2895
Xu L et al. (2004) Results of a phase II trial of Herceptin® plus Xeloda® in patients with previously untreated HER2-positive metastatic breast cancer [abstract #3049]. Breast Cancer Res Treat 88 (Suppl 1): S128–S129
Burris H 3rd et al. (2004) Phase II trial of trastuzumab followed by weekly pacliatxel/carboplatin as first-line treatment for metastatic breast cancer. J Clin Oncol 22: 1621–1629
Perez EA et al. (2005) Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer 6: 425–432
Robert NJ et al. (2006) Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2 overexpressing metastatic breast cancer: an update including survival. J Clin Oncol 24: 2786–2792
Yardley DA et al. (2004) First line treatment with weekly docetaxel, vinorelbine, and trastuzumab in HER2 overexpressing metastatic breast cancer (HER2+ MBC): a Minnie Pearl Cancer Research Network phase II trial. J Clin Oncol 22 (Suppl 14): 643a
O'Shaughnessy JA et al. (2004) Phase II study of trastuzumab plus gemcitabine in chemotherapy-pretreated patients with metastatic breast cancer. Clin Breast Cancer 5: 142–147
Miller KD et al. (2002) Phase II study of gemcitabine, paclitaxel and trastuzumab in metastatic breast cancer: a Hossier Oncology Group study [abstract #437]. Breast Canc Res Treat 76 (Suppl): S113
Hortobagyi GN (2005) Trastuzumab in the treatment of breast cancer. N Engl J Med 20 1734–1736
Pegram M et al. (1999) Inhibitory effects of combination of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18: 2241–2251
Pietras RJ et al. (1998) Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER2 receptor and DNA-reactive drugs. Oncogene 17: 2235–2249
Smith I et al. (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369: 29–36
Slamon D et al. (2006) BCIRG 006: 2nd interim analysis Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC → T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC → TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2/neu positive early breast cancer patients. Breast Cancer Res Treat 106 (Suppl 1): 52a
NCCN (2008) NCCN Clinical Practice Guidelines in Oncology version 2: breast cancer. [http://www.nccn.org] (accessed May 2008)
Perez EA et al. (2004) Clinical cardiac tolerability of trastuzumab. J Clin Oncol 22: 322–239
Leyland-Jones B et al. (2003) Pharmacokinetics, safety and efficacy of trastuzumab administered every three weeks in combinations with paclitaxel. J Clin Oncol 21: 3965–3971
Cobleigh MA et al. (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17: 2639–2648
Vogel CL et al. (2002) Efficacy and safety of trastuzumab as single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20: 719–726
Baselga J et al. (2005) Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol 23: 2162–2171
Warrel D et al. (Eds) (1997) Oxford Textbook of Medicine Vol 2, pp 2228. Oxford University Press.
Perez EA et al. (2004) Effect of doxorubicin plus cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J Clin Oncol 15: 3700–3704
Perez EA et al. (2005) Exploratory analysis from NCCTG N9831: do clinical and laboratory characteristics predict cardiac toxicity of trastuzumab when administered as a component of adjuvant therapy? Breast Cancer Res Treat 94 (Suppl): S2038
Tan-Chiu E et al. (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23: 7811–7819
Telli ML et al. (2007) Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 25: 3525–3533
Ewer MS et al. (2005) Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 23: 7820–7826
Chien KR (2006) Herceptin and the heart—a molecular modifier of cardiac failure. N Engl J Med 23: 789–790
Ewer MS et al. (2005) Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 23: 2900–2902
Citri A et al. (2006) EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7: 505–516
Gianni L et al. (2007) Anthracycline cardiotoxicity in breast cancer patients: synergism with trastuzumab and taxanes. Cardiovasc Toxicol 7: 67–71
Lee KF et al. (1995) Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378: 394–398
Crone SA et al. (2002) ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8: 459–465
De Alava E et al. (2007) Neuregulin expression modulates clinical response to trastuzumab in patients with metastatic breast cancer. J Clin Oncol 25: 2656–2663
Menendez JA et al. (2006) Trastuzumab in combination with heregulin-activated Her-2 (erbB-2) triggers a receptor-enhanced chemosensitivity effect in the absence of Her-2 overexpression. J Clin Oncol 24: 3735–3746
Yuste L et al. (2005) Activation of ErbB2 by overexpression or by transmembrane neuregulin results in differential signaling and sensitivity to herceptin. Cancer Res 65: 6801–6810
Arteaga CL (2006) Can trastuzumab be effective against tumors with low HER2/Neu (ErbB2) receptors? J Clin Oncol 24: 3722–3725
Moy B et al. (2006) Lapatinib: current status and future directions in breast cancer. Oncologist 11: 1047–1057
Piccart-Gebhart MJ et al. (2007) ALTTO (adjuvant lapatinib and/or trastuzumab treatment optimisation) study (BIG 2-06/N063D/EGF106708): A phase III study for HER-2 overexpressing early breast cancer [abstract #118]. Breast 16 (Suppl): S46–S47
Moy B et al. (2007) Phase III study of lapatinib after completion of adjuvant therapy in trastuzumab-naïve women with ErbB2-overexpressing breast cancer: Tykerb evaluation after chemotherapy (TEACH) study [abstract #119]. Breast 16 (Suppl): S47
Moy B et al. (2007) TEACH: Tykerb evaluation after chemotherapy. Clin Breast Cancer 7: 489–492
Cortes J et al. (2005) Open label, randomized, phase II study of pertuzumab (P) in patients (pts) with metastatic breast cancer (MBC) with low expression of HER2 [abstract #3068]. J Clin Oncol 23 (Suppl 16S)
Baselga J et al. (2007) Objective response rate in a phase II multicenter trial of pertuzumab (P), a HER2 dimerization inhibiting monoclonal antibody, in combination with trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) which has progressed during treatment with T [abstract #1004]. J Clin Oncol 24 (Suppl 18S)
Musolino A et al. (2008) Immunoglobulin G fragment C receptor polymorphisms and response to trastuzumab-based treatment in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 26: 1778–1780
Scaltriti M et al. (2007) Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 18: 628–638
Yakes FM et al. (2002) Herceptin-induced inhibition of phosphatidylinositol 3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 62: 4132–4141
Nagata Y et al. (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6: 117–127
Nahta R et al. (2005) Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65: 11118–11128
Cardoso F et al. (2002) Resistance to trastuzumab: a necessary evil or a temporary challenge? Clin Breast Cancer 3: 247–257
Nahta R et al. (2006) Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 3: 269–280
Kim C et al. (2005) Trastuzumab sensitivity of breast cancer with co-amplification of HER2 and cMYC suggests pro-apoptotic function of dysregulated cMYC in vivo. Breast Cancer Res Treat 94: S46.
Jarvinen TA et al. (2000) Amplification and deletion of topoisomerase IIalpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol 156: 839–847
Park K et al. (2003) Topoisomerase II-alpha (topoII) and HER2 amplification in breast cancers and response to preoperative doxorubicin chemotherapy. Eur J Cancer 39: 631–634
Ross JS et al. (2003) The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 8: 307–325
Fritz P et al. (2005) c-erbB2 and topoisomerase II alpha protein expression independently predict poor survival in primary human breast cancer: a retrospective study. Breast Cancer Res 7: 374–384
Press MF et al. (2005) Topoisomerase II-alpha gene amplification as a predictor of responsiveness to anthracycline-containing chemotherapy in the Cancer International Research Group 006 clinical trial of trastuzumab (Herceptin) in the adjuvant setting [abstract #1045]. Breast Cancer Res Treat 94 (Suppl): S54
Leyland-Jones B (2001) Dose Scheduling-Herceptin®. Oncology 61: 31–36
Buzdar AU et al. (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23: 3676–3685
Buzdar AU et al. (2007) Neoadjuvant therapy with paclitaxel followed by FEC chemotherapy and concurrent trastuzumab in HER2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 13: 228–233
Gianni L et al. (2007) Neoadjuvant trastuzumab plus doxorubicin, paclitaxel and CMF in locally advanced breast cancer (NOAH trial): feasibility, safety and antitumor effects [abstract #144]. 2007 Breast Cancer Symposium
Neyt M et al. (2005) An economic evaluation of Herceptin® in adjuvant setting: the Breast Cancer International Research Group 006 trial. Ann Oncol 17: 381–390
Gianni L (2005) The cost of life: should it matter to doctors? Ann Oncol 17: 357–358
Garrison LP Jr et al. (2007) Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer 110: 489–498
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Luca Gianni declared he is a consultant for Genentech, GlaxoSmithKline, Roche and Wyeth. The other authors declared no competing interests.
Rights and permissions
About this article
Cite this article
Mariani, G., Fasolo, A., De Benedictis, E. et al. Trastuzumab as adjuvant systemic therapy for HER2-positive breast cancer. Nat Rev Clin Oncol 6, 93–104 (2009). https://doi.org/10.1038/ncponc1298
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1298
This article is cited by
-
Transformable peptide nanoparticles arrest HER2 signalling and cause cancer cell death in vivo
Nature Nanotechnology (2020)
-
Incidence and identification of risk factors for trastuzumab-induced cardiotoxicity in breast cancer patients: an audit of a single “real-world” setting
Medical Oncology (2017)
-
Bioorthogonal two-component drug delivery in HER2(+) breast cancer mouse models
Scientific Reports (2016)
-
Transcriptomic analysis of human breast cancer cells reveals differentially expressed genes and related cellular functions and pathways in response to gold nanorods
Biophysics Reports (2015)
-
Effectiveness of neoadjuvant trastuzumab and chemotherapy in HER2-overexpressing breast cancer
Journal of Cancer Research and Clinical Oncology (2013)