Abstract
Since 2004, four antiangiogenic drugs have been approved for clinical use in patients with advanced solid cancers, on the basis of their capacity to improve survival in phase III clinical studies. These achievements validated the concept introduced by Judah Folkman that the inhibition of tumor angiogenesis could control tumor growth. It has been suggested that biomarkers of angiogenesis would greatly facilitate the clinical development of antiangiogenic therapies. For these four drugs, the pharmacodynamic effects observed in early clinical studies were important to corroborate activities, but were not essential for the continuation of clinical development and approval. Furthermore, no validated biomarkers of angiogenesis or antiangiogenesis are available for routine clinical use. Thus, the quest for biomarkers of angiogenesis and their successful use in the development of antiangiogenic therapies are challenges in clinical oncology and translational cancer research. We review critical points resulting from the successful clinical trials, review current biomarkers, and discuss their potential impact on improving the clinical use of available antiangiogenic drugs and the development of new ones.
Key Points
-
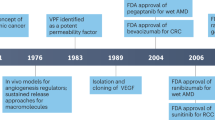
Four antiangiogenic drugs, bevacizumab, sorafenib, sunitinib and temsirolimus, have been approved for clinical use on the basis of results from randomized phase III clinical trials without significant contributions from biomarkers
-
No validated biomarkers of angiogenesis or antiangiogeneic activity are available for routine clinical use
-
Biomarkers of angiogenesis might be useful for monitoring angiogenesis, assessing drug activity and distinguishing between active and inactive drugs, predicting clinical outcome and response to therapy, defining the optimum biological dose, facilitating development of combination therapies, and rapidly identifying resistance to treatment
-
Biomarkers under consideration for clinical use include circulating cells, proteins (e.g. angiogenic factors, angiogenesis-associated molecules; protein expression profiles), nucleic acids (e.g. gene-expression patterns) and functional parameters (e.g. tumor perfusion, metabolism)
-
The association of laboratory investigations with clinical trials will be instrumental for the validation of biomarkers of angiogenesis and for improving the design, monitoring and evaluation of antiangiogenic treatments
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438: 932–936
Ferrara N and Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature 438: 967–974
Kerbel R and Folkman J (2002) Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2: 727–739
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25: 581–611
Ferrara N et al. (2005) Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 333: 328–335
Rini BI (2006) Sorafenib. Expert Opin Pharmacother 7: 453–461
Motzer RJ et al. (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24: 16–24
Dancey JE (2005) Inhibitors of the mammalian target of rapamycin. Expert Opin Investig Drugs 14: 313–328
Jain RK et al. (2006) Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 3: 24–40
Therasse P (2002) Measuring the clinical response—what does it mean? Eur J Cancer 38: 1817–1823
Schilsky RL (2002) End points in cancer clinical trials and the drug approval process. Clin Cancer Res 8: 935–938
Willett CG et al. (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147
Escudier B et al. (2007) Randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-alpha2a vs placebo/interferon-alpha2a as first-line therapy in metastatic renal cell carcinoma [abstract #3]. J Clin Oncol 25 (Suppl) S18
Hurwitz H et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342
Mass RD et al. (2005) Clinical benefit from bevacizumab (BV) in responding (R) and non-responding (NR) patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 23: S249–S249
Panares RL and Garcia AA (2007) Bevacizumab in the management of solid tumors. Expert Rev Anticancer Ther 7: 433–445
Ratain MJ et al. (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24: 2505–2512
Escudier B et al. (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134
Bukowski RM et al. (2007) Final results of the randomized phase III trial of sorafenib in advanced renal cell carcinoma: survival and biomarker analysis [abstract #5023]. J Clin Oncol 25 (Suppl): S18
Chow LQ and Eckhardt SG (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25: 884–896
Motzer RJ et al. (2006) Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295: 2516–2524
Motzer RJ et al. (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124
George DJ et al. (2007) Phase II trial of sunitinib in bevacizumab-refractory metastatic renal cell carcinoma (mRCC): updated results and analysis of circulating biomarkers [abstract #5053]. J Clin Oncol 25 (Suppl): S18
Bjornsti MA and Houghton PJ (2004) The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4: 335–348
Atkins MB et al. (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22: 909–918
Hudes G et al. (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356: 2271–2281
Morgan B et al. (2003) Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol 21: 3955–3964
Kim WY and Kaelin WG Jr (2006) Molecular pathways in renal cell carcinoma—rationale for targeted treatment. Semin Oncol 33: 588–595
Ludwig JA and Weinstein JN (2005) Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 5: 845–856
Rüegg C et al. (2003) The quest for surrogate markers of angiogenesis: a paradigm for translational research in tumor angiogenesis and anti-angiogenesis trials. Curr Mol Med 3: 673–691
Davis DW et al. (2003) Surrogate markers in antiangiogenesis clinical trials. Br J Cancer 89: 8–14
Bhatt RS et al. (2007) Biomarkers for monitoring antiangiogenic therapy. Clin Cancer Res 13: S777–S780
Poon RT et al. (2001) Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol 19: 1207–1225
Jubb AM et al. (2006) Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 24: 217–227
Bocci G et al. (2004) Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res 64: 6616–6625
Willett CG et al. (2005) Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol 23: 8136–8139
Zaman K et al. (2006) Monitoring multiple angiogenesis-related molecules in the blood of cancer patients shows a correlation between VEGF-A and MMP-9 levels before treatment and divergent changes after surgical vs conservative therapy. Int J Cancer 118: 755–764
Hlatky L et al. (2002) Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst 94: 883–893
Solorzano CC et al. (2001) In vivo intracellular signaling as a marker of antiangiogenic activity. Cancer Res 61: 7048–7051
Bertolini F et al. (2006) The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 6: 835–845
Mancuso P et al. (2001) Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 97: 3658–3661
Shaked Y et al. (2005) Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood 106: 3058–3061
Mancuso P et al. (2006) Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 108: 452–459
Beaudry P et al. (2005) Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res 11: 3514–3522
Norden-Zfoni A et al. (2007) Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res 13: 2643–2650
Strumberg D et al. (2005) Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 23: 965–972
Mittal V and Nolan DJ (2007) Genomics and proteomics approaches in understanding tumor angiogenesis. Expert Rev Mol Diagn 7: 133–147
St Croix B et al. (2000) Genes expressed in human tumor endothelium. Science 289: 1197–1202
Hardwick JS et al. (2005) Identification of biomarkers for tumor endothelial cell proliferation through gene expression profiling. Mol Cancer Ther 4: 413–425
Abe M and Sato Y (2001) cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis 4: 289–298
van Beijnum JR and Griffioen AW (2005) In silico analysis of angiogenesis associated gene expression identifies angiogenic stage related profiles. Biochim Biophys Acta 1755: 121–134
Smirnov DA et al. (2006) Global gene expression profiling of circulating endothelial cells in patients with metastatic carcinomas. Cancer Res 66: 2918–2922
Rabascio C et al. (2004) Assessing tumor angiogenesis: increased circulating VE-cadherin RNA in patients with cancer indicates viability of circulating endothelial cells. Cancer Res 64: 4373–4377
DePrimo SE et al. (2003) Expression profiling of blood samples from an SU5416 phase III metastatic colorectal cancer clinical trial: a novel strategy for biomarker identification. BMC Cancer 3: 3
Abdollahi A et al. (2004) Endostatin's antiangiogenic signaling network. Mol Cell 13: 649–663
Ayers M et al. (2007) Discovery and validation of biomarkers that respond to treatment with brivanib alaninate, a small-molecule VEGFR-2/FGFR-1 antagonist. Cancer Res 6: 6899–6906
Mustafa DA et al. (2007) Identification of glioma neovascularization-related proteins by using MALDI-FTMS and nano-LC fractionation to microdissected tumor vessels. Mol Cell Proteomics 6: 1147–1157
Celis JE et al. (2004) Proteomic characterization of the interstitial fluid perfusing the breast tumor microenvironment: a novel resource for biomarker and therapeutic target discovery. Mol Cell Proteomics 3: 327–344
Bruneel A et al. (2003) Proteomic study of human umbilical vein endothelial cells in culture. Proteomics 3: 714–723
Pellitteri-Hahn MC et al. (2006) Improved mass spectrometric proteomic profiling of the secretome of rat vascular endothelial cells. J Proteome Res 5: 2861–2864
Godl K et al. (2005) Proteomic characterization of the angiogenesis inhibitor SU6668 reveals multiple impacts on cellular kinase signaling. Cancer Res 65: 6919–6926
Campostrini N et al. (2006) Proteomic analysis of anti-angiogenic effects by a combined treatment with vinblastine and rapamycin in an endothelial cell line. Proteomics 6: 4420–4431
Alessandro R et al. (2005) Proteomic approaches in colon cancer: promising tools for new cancer markers and drug target discovery. Clin Colorectal Cancer 4: 396–402
Pan S et al. (2005) High throughput proteome screening for biomarker detection. Mol Cell Proteomics 4: 182–190
Ocak I et al. (2007) The biologic basis of in vivo angiogenesis imaging. Front Biosci 12: 3601–3616
Provenzale JM (2007) Imaging of angiogenesis: clinical techniques and novel imaging methods. AJR Am J Roentgenol 188: 11–23
Cai W et al. (2006) How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther 5: 2624–2633
Wintermark M et al. (2001) Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol 11: 1220–1230
Atri M (2006) New technologies and directed agents for applications of cancer imaging. J Clin Oncol 24: 3299–3308
Miller JC et al. (2005) Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst 97: 172–187
Rosen MA and Schnall MD (2007) Dynamic contrast-enhanced magnetic resonance imaging for assessing tumor vascularity and vascular effects of targeted therapies in renal cell carcinoma. Clin Cancer Res 13: S770–S776
Leach MO et al. (2005) The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 92: 1599–1610
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7: 91–101
Miles KA (2003) Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol 76: S36–S42
Goh V et al. (2007) Quantitative tumor perfusion assessment with multidetector CT: are measurements from two commercial software packages interchangeable? Radiology 242: 777–782
Roberts HC et al. (2002) Dynamic, contrast-enhanced CT of human brain tumors: quantitative assessment of blood volume, blood flow, and microvascular permeability: report of two cases. AJNR Am J Neuroradiol 23: 828–832
Sahani DV et al. (2005) Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology 234: 785–792
Raichle ME et al. (1983) Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med 24: 790–798
Sadeghi N et al. (2006) Correlation between dynamic susceptibility contrast perfusion MRI and methionine metabolism in brain gliomas: preliminary results. J Magn Reson Imaging 24: 989–994
Padhani A (2006) PET imaging of tumour hypoxia. Cancer Imaging 6: S117–S121
Phillips P and Gardner E (2004) Contrast-agent detection and quantification. Eur Radiol 14 (Suppl 8): S4–S10
Wei K et al. (1998) Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97: 473–483
Broillet A et al. (2005) Assessment of microvascular perfusion changes in a rat breast tumor model using SonoVue to monitor the effects of different anti-angiogenic therapies. Acad Radiol 12 (Suppl 1): S28–S33
Lucidarme O et al. (2006) Angiogenesis: noninvasive quantitative assessment with contrast-enhanced functional US in murine model. Radiology 239: 730–739
Lassau N et al. (2006) Prognostic value of angiogenesis evaluated with high-frequency and colour Doppler sonography for preoperative assessment of primary cutaneous melanomas: correlation with recurrence after a 5-year follow-up period. Cancer Imaging 6: 24–29
Lamuraglia M et al. (2006) To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer 42: 2472–2479
Fury MG et al. (2007) A Phase II study of SU5416 in patients with advanced or recurrent head and neck cancers. Invest New Drugs 25: 165–172
Schirner M et al. (2004) Molecular imaging of tumor angiogenesis. Ann NY Acad Sci 1014: 67–75
Casanovas O et al. (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8: 299–309
Shaked Y et al. (2006) Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 313: 1785–1787
Arditi M et al. (2006) A new formalism for the quantification of tissue perfusion by the destruction-replenishment method in contrast ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control 53: 1118–1129
Dark GG et al. (1997) Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res 57: 1829–1834
Stevenson JP et al. (2003) Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol 21: 4428–4438
Yang JC et al. (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434
Acknowledgements
We thank Drs F Bosman, A Mariotti, M Arditi and R Meuli for helpful comments and discussions, Dr JY Mewly for providing radiological images and R Driscoll for proofreading the manuscript. We apologize to those colleagues whose work could not be cited owing to space limitations. Our research activities are supported by grants from the Swiss National Science Foundation (SNF), Oncosuisse, the National Center for Competence in Research (NCCR, molecular Oncology, a research instrument of the SNF), the Medic Foundation and the Southern European New Drugs Organization (SENDO) Foundation. Désirée Lie, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sessa, C., Guibal, A., Del Conte, G. et al. Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: tools or decorations?. Nat Rev Clin Oncol 5, 378–391 (2008). https://doi.org/10.1038/ncponc1150
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1150
This article is cited by
-
Implications of flavonoids as potential modulators of cancer neovascularity
Journal of Cancer Research and Clinical Oncology (2020)
-
Cancer nanomedicine: progress, challenges and opportunities
Nature Reviews Cancer (2017)
-
Novel affinity binders for neutralization of vascular endothelial growth factor (VEGF) signaling
Cellular and Molecular Life Sciences (2016)
-
Blood-based biomarkers for monitoring antiangiogenic therapy in non-small cell lung cancer
Medical Oncology (2016)
-
A network model for angiogenesis in ovarian cancer
BMC Bioinformatics (2015)