Abstract
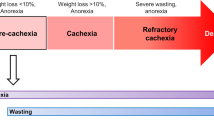
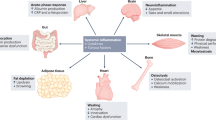
Tumor growth is associated with profound metabolic and neurochemical alterations, which can lead to the onset of anorexia–cachexia syndrome. Anorexia is defined as the loss of the desire to eat, while cachexia results from progressive wasting of skeletal muscle mass—and to a lesser extent adipose tissue—occurring even before weight loss becomes apparent. Cancer anorexia–cachexia syndrome is highly prevalent among cancer patients, has a large impact on morbidity and mortality, and impinges on patient quality of life. However, its clinical relevance is frequently overlooked, and treatments are usually only attempted during advanced stages of the disease. The pathogenic mechanisms of cachexia and anorexia are multifactorial, but cytokines and tumor-derived factors have a significant role, thereby representing a suitable therapeutic target. Energy expenditure in anorexia is frequently increased while energy intake is decreased, which further exacerbates the progressive deterioration of nutritional status. The optimal therapeutic approach to anorectic–cachectic cancer patients should be based on both changes in dietary habits, achieved via nutritional counseling; and drug therapy, aimed at interfering with cytokine expression or activity. Our improved understanding of the influence a tumor has on the host's metabolism is advancing new therapeutic approaches, which are likely to result in better preservation of nutritional status if started concurrently with specific antineoplastic treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ayers M et al. (2004) Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J Clin Oncol 22: 2284–2293
Tisdale MJ (2002) Cachexia in cancer patients. Nat Rev Cancer 2: 862–871
Bossola M et al. (2001) Increased muscle ubiquitin mRNA levels in gastric cancer patients. Am J Physiol Regul Integr Comp Physiol 280: R1518–R1523
Bozzetti F et al. (1999) Artificial nutrition in cancer patients: which route, what composition? World J Surg 23: 577–583
Norton JA et al. (1981) Whole body protein synthesis and turnover in normal man and malnourished patients with and without known cancer. Ann Surg 194: 123–128
Lundholm K et al. (1982) Efflux of 3-methylhistidine from the leg in cancer patients who experience weight loss. Cancer Res 42: 4802–4811
Lundholm K et al. (1976) Skeletal muscle metabolism in patients with malignant tumour. Eur J Cancer 12: 465–473
Warren RS et al. (1985) Protein synthesis in the tumor-influenced hepatocyte. Surgery 98: 275–282
Lecker SH et al. (1999) Muscle protein breakdown and critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129 (Suppl 1S): 227S–237S
Goll DE et al. (1992) Role of the calpain system in muscle growth. Biochimie 74: 225–237
Drott C et al. (1989) Cardiovascular and metabolic response to adrenaline infusion in weight-losing patients with and without cancer. Clin Physiol 9: 427–439
Bing C et al. (2000) Increased gene expression of brown fat uncoupling protein (UCP)1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia. Cancer Res 60: 2405–2410
Todorov P et al. (1996) Characterization of a cancer cachectic factor. Nature 379: 739–742
Russell ST et al. (2002) Role of β3-adrenergic receptors in the action of a tumour lipid mobilizing factor. Br J Cancer 86: 424–428
Ramos EJ et al. (2004) Cancer anorexia–cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care 7: 427–434
Schwartz MW et al. (2000) Central nervous system control of food intake. Nature 404: 661–671
Laviano A et al. (2003) Cancer anorexia: clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol 4: 686–694
Stubbs RJ et al. (2000) The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 84: 405–415
Rossi Fanelli F et al. (1986) Plasma tryptophan and anorexia in human cancer. Eur J Cancer Clin Oncol 22: 89–95
Muscaritoli M et al. (2004) Therapy of muscle wasting: what is the future? Curr Opin Clin Nutr Metab Care 7: 459–466
Bruera E et al. (2003) Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J Clin Oncol 21: 129–134
Laviano A and Meguid MM (1996) Nutritional issues in cancer management. Nutrition 12: 358–371
Geels P et al. (2000) Palliative effect of chemotherapy: objective tumor response is associated with symptom improvement in patients with metastatic breast cancer. J Clin Oncol 18: 2395–2405
Sutton LM et al. (2003) Management of terminal cancer in elderly patients. Lancet Oncol 4: 149–157
Walsh D et al. (2000) The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 8: 175–179
DeWys WD et al. (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 69: 491–497
Palesty JA and Dudrick SJ (2003) What we have learned about cachexia in gastrointestinal cancer. Dig Dis 21: 198–213
Ruiz-Arguelles GJ et al. (2004) Multiple myeloma in Mexico: a 20-year experience at a single institution. Arch Med Res 35: 163–167
Thammakumpee K (2004) Clinical manifestation and survival of patients with non-small cell lung cancer. J Med Assoc Thai 87: 503–507
Walsh D et al. (2002) Symptoms and prognosis in advanced cancer. Support Care Cancer 10: 385–388
Ravasco P et al. (2004) Cancer: disease and nutrition are key determinants of patients' quality of life. Support Care Cancer 12: 246–252
Cherny NI and Catane R (2003) Attitudes of medical oncologists toward palliative care for patients with advanced and incurable cancer: report on a survey by the European Society of Medical Oncology Taskforce on Palliative and Supportive Care. Cancer 98: 2502–2510
Isenring EA et al. (2004) Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer 91: 447–452
Feinle C et al. (2003) Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol 284: G798–G807
Inui A (2002) Cancer anorexia–cachexia syndrome: current issues in research and management. CA Cancer J Clin 52: 72–91
Pascual Lopez A et al. (2004) Systematic review of megestrol acetate in the treatment of anorexia–cachexia syndrome. J Pain Symptom Manage 27: 360–369
Jho DH et al. (2004) Role of omega-3 fatty acid supplementation in inflammation and malignancy. Integr Cancer Ther 3: 98–111
Fearon KC et al. (2003) Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 52: 1479–1486
Moses AW et al. (2004) Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer 90: 996–1002
Jatoi A et al. (2004) An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 22: 2469–2476
Burns CP et al. (2004) Phase II study of high-dose fish oil capsules for patients with cancer-related cachexia. Cancer 101: 370–378
Torelli GF et al. (1999) Use of recombinant human soluble TNF receptor in anorectic tumor-bearing rats. Am J Physiol 277: R850–R855
Trikha M et al. (2003) Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res 9: 4653–4665
Eleutherakis-Papaiakovou V et al. (2004) Thalidomide in cancer medicine. Ann Oncol 15: 1151–1160
Eichhorst ST et al. (2004) Suramin inhibits death receptor-induced apoptosis in vitro and fulminant apoptotic liver damage in mice. Nat Med 10: 602–609
Diksic M and Young SN (2001) Study of the brain serotonergic system with labelled α-methyl-L-tryptophan. J Neurochem 78: 1185–1200
Cangiano C et al. (1996) Effects of administration of oral branched-chain amino acids on anorexia and caloric intake in cancer patients. J Natl Cancer Inst 88: 550–552
Hiroshige K et al. (2001) Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol Dial Transplant 16: 1856–1862
Marchesini G et al. for the Italian BCAA Study Group. (2003) Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 124: 1792–1801
Heisler LK et al. (2002) Activation of central melanocortin pathways by fenfluramine. Science 297: 609–611
Marks DL et al. (2003) Differential role of melanocortin receptor subtypes in cachexia. Endocrinology 144: 1513–1523
Smith HJ et al. (2004) Effect of eicosapentaenoic acid, protein and amino acids on protein synthesis and degradation in skeletal muscle of cachectic mice. Br J Cancer 91: 408–412
Inui A et al. (2004) Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 18: 439–456
Shimizu Y et al. (2003) Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res 9: 774–778
Neary NM et al. (2004) Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab 89: 2832–2836
Cahlin C et al. (2000) Effect of cyclooxygenase and nitric oxide synthase inhibitors on tumor growth in mouse tumor models with and without cachexia related to prostanoids. Cancer Res 60: 1742–1749
Lundholm K et al. (2004) Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: effects on survival, metabolism, and function. Cancer 100: 1967–1977
Inui A (1999) Cancer anorexia–cachexia syndrome: are neuropeptides the key? Cancer Res 59: 4493–4501
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
F Rossi-Fanelli received a research grant from the Ross Products Division of Abbott Laboratories, Columbus, Ohio, USA. Abbott Laboratories produces a caloric supplement enriched with fish oil.
Rights and permissions
About this article
Cite this article
Laviano, A., Meguid, M., Inui, A. et al. Therapy Insight: cancer anorexia–cachexia syndrome—when all you can eat is yourself. Nat Rev Clin Oncol 2, 158–165 (2005). https://doi.org/10.1038/ncponc0112
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc0112
This article is cited by
-
Vacuolar protein sorting 35 (VPS35) acts as a tumor promoter via facilitating cell cycle progression in pancreatic ductal adenocarcinoma
Functional & Integrative Genomics (2023)
-
Plasma Inflammatory Biomarkers and Anorexia of Ageing among Community-Dwelling Older Adults: An Exploratory Analysis of the MAPT Study
The Journal of nutrition, health and aging (2023)
-
Cannabinoids orchestrate cross-talk between cancer cells and endothelial cells in colorectal cancer
Cancer Gene Therapy (2022)
-
Fasting-Mimicking-Diet does not reduce skeletal muscle function in healthy young adults: a randomized control trial
European Journal of Applied Physiology (2022)
-
Effects of whole-body vibration training in a cachectic C26 mouse model
Scientific Reports (2021)