Abstract
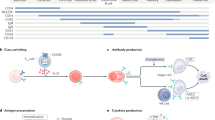
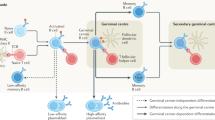
B cells have a fundamental role in the pathogenesis of various autoimmune neurological disorders, not only as precursors of antibody-producing cells, but also as important regulators of the T-cell activation process through their participation in antigen presentation, cytokine production, and formation of ectopic germinal centers in the intermeningeal spaces. Two B-cell trophic factors—BAFF (B-cell-activating factor) and APRIL (a proliferation-inducing ligand)—and their receptors are strongly upregulated in many immunological disorders of the CNS and PNS, and these molecules contribute to clonal expansion of B cells in situ. The availability of monoclonal antibodies or fusion proteins against B-cell surface molecules and trophic factors provides a rational approach to the treatment of autoimmune neurological diseases. This article reviews the role of B cells in autoimmune neurological disorders and summarizes the experience to date with rituximab, a B-cell-depleting monoclonal antibody against CD20, for the treatment of relapsing–remitting multiple sclerosis, autoimmune neuropathies, neuromyelitis optica, paraneoplastic neurological disorders, myasthenia gravis, and inflammatory myopathies. It is expected that ongoing controlled trials will establish the efficacy and long-term safety profile of anti-B-cell agents in several autoimmune neurological disorders, as well as exploring the possibility of a safe and synergistic effect with other immunosuppressants or immunomodulators.
Key Points
-
B cells have a key role in the pathogenesis of various autoimmune neurological disorders
-
A number of monoclonal antibodies or fusion proteins directed against B-cell surface molecules and trophic factors are currently in clinical trials
-
The anti-CD20 monoclonal antibody rituximab has shown promise in the treatment of disorders such as relapsing–remitting multiple sclerosis, autoimmune neuropathies, neuromyelitis optica, paraneoplastic neurological disorders, stiff-person syndrome, myasthenia gravis and inflammatory myopathies
-
Circulating B cells, but not the antibody-producing plasma cells that do not express CD20, become undetec Table 1 month after rituximab treatment, and levels remain low for at least 6 months
Table 1 B-cell trophic factors and their receptors in autoimmune neurological disorders. -
Despite B-cell depletion, patients do not seem to be prone to common infections after rituximab treatment
-
Experience in conditions such as vasculitis and rheumatoid arthritis indicates that rituximab can be used in combination with other immunosuppressants
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hasler P and Zouali M (2006) B lymphocytes as therapeutic targets in systemic lupus erythematosus. Expert Opin Ther Targets 10: 803–815
Shlomchik MJ et al. (2001) From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol 1: 147–153
Dalakas MC (2008) Invited article: inhibition of B cell functions: implications for neurology. Neurology 70: 2252–2260
Browning JL (2006) B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov 5: 564–576
Goldsby RA et al. (2000) Kuby Immunology, edn 4. New York: WH Freeman and Company
Martin F and Chan AC (2006) B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol 24: 467–496
Yurasov S et al. (2005) Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 201: 703–711
Chan OT et al. (1999) A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med 189: 1639–1648
Lanzavecchia A (1990) Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol 8: 773–793
Lund FE et al. (2005) Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun 8: 25–54
Duddy ME et al. (2004) Distinct profiles of human B cell effector cytokines: a role in immune regulation. J Immunol 172: 3422–3427
Knopf PM et al. (1998) Antigen-dependent intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen-specific B cells. J Immunol 161: 692–701
Alter A et al. (2003) Determinants of human B cell migration across brain endothelial cells. J Immunol 170: 4497–4505
Meinl E et al. (2006) B lineage cells in the inflammatory central nervous system environment; migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol 59: 880–892
Ritchie AM et al. (2004) Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol 173: 649–656
Serafini B et al. (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14: 164–174
Magliozzi R et al. (2007) Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130: 1089–1104
Dillon SR et al. (2006) An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov 5: 235–246
Peter HH and Warnatz K (2005) Molecules involved in T–B co-stimulation and B cell homeostasis: possible targets for an immunological intervention in autoimmunity. Expert Opin Biol Ther 5 (Suppl 1): S61–S71
Thangarajh M et al. (2005) Increased levels of APRIL (a proliferation-inducing ligand) mRNA in multiple sclerosis. J Neuroimmunol 167: 210–214
Krumbholz M et al. (2005) BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med 201: 195–200
Thangarajh M et al. (2007) A proliferation-inducing ligand (APRIL) is expressed by astrocytes and is increased in multiple sclerosis. Scand J Immunol 65: 92–98
Kalled SL (2006) Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol 18: 290–296
Stohl W (2004) Targeting B lymphocyte stimulator in systemic lupus erythematosus and other autoimmune rheumatic disorders. Expert Opin Ther Targets 8: 177–189
Huntington ND et al. (2006) A BAFF antagonist suppresses experimental autoimmune encephalomyelitis by targeting cell-mediated and humoral immune responses. Int Immunol 18: 1473–1485
Eisenberg R and Albert D (2006) B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol 2: 20–27
Edwards JC and Cambridge G (2005) Prospects for B-cell-targeted therapy in autoimmune disease. Rheumatology (Oxford) 44: 151–156
Lucchinetti C et al. (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47: 707–717
Frohman EM et al. (2006) Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354: 942–955
Cepok S et al. (2005) Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain 128: 1667–1676
Genain CP et al. (1999) Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 5: 170–175
Monson NL et al. (2005) Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol 62: 258–264
Hauser S et al. (2008) B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med 358: 676–688
Bar-Or A et al. (2008) Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 63: 395–400
Jarius S et al. (2008) Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol 4: 202–214
Cree BA and Wingerchuk DM (2005) Acute transverse myelitis: is the “idiopathic” form vanishing. Neurology 65: 1857–1858
Jacob A et al. (2007) Retrospective analysis of rituximab treatment of 24 patients with neuromyelitis optica [abstract #S32.002]. Neurology 68 (Suppl 1): A206
Genain C et al. (2007) An open label clinical trial of rituximab in neuromyelitis optica [abstract #S32.001]. Neurology 68 (Suppl 1): A205
Pranzatelli MR et al. (2004) B- and T-cell markers in opsoclonus–myoclonus syndrome: immunophenotyping of CSF lymphocytes. Neurology 62: 1526–1532
Pranzatelli MR et al. (2006) Rituximab (anti-CD20) adjunctive therapy for opsoclonus–myoclonus syndrome. J Pediatr Hematol Oncol 28: 585–593
Dalakas M and Engel WK (1980) Immunoglobulin and complement deposits in nerves of patients with chronic relapsing polyneuropathy. Arch Neurol 37: 637–640
Hays AP et al. (1988) Immune reactive C3d on the surface of myelin sheaths in neuropathy. J Neuroimmunol 18: 231–244
Pestronk A et al. (2003) Treatment of IgM antibody associated polyneuropathies using rituximab. J Neurol Neurosurg Psychiatry 74: 485–489
Ruegg SJ et al. (2004) Rituximab stabilizes multifocal motor neuropathy increasingly less responsive to IVIg. Neurology 63: 2178–2179
Levine TD and Pestronk A (1999) IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using rituximab. Neurology 52: 1701–1704
Renaud S et al. (2003) Rituximab in the treatment of polyneuropathy associated with anti-MAG antibodies. Muscle Nerve 27: 611–615
Dalakas MC et al. (2007) A double-blind placebo-controlled study of rituximab in patients with anti-MAG antibody-demyelinating polyneuropathy (A-MAG-DP) [abstract #S38.001]. Neurology 68 (Suppl 1): A214
Raju R et al. (2006) Autoimmunity to GABAA-receptor-associated protein in stiff-person syndrome. Brain 129: 3270–3276
Dalakas MC et al. (2001) Stiff-person syndrome: quantification, specificity and intrathecal synthesis of GAD65 antibodies. Neurology 57: 780–785
Baker MR et al. (2005) Treatment of stiff person syndrome with rituximab. J Neurol Neurosurg Psychiatry 76: 999–1001
Dalakas MC and Hohlfeld R (2003) Polymyositis and dermatomyositis. Lancet 362: 971–982
Noss EH et al. (2006) Rituximab as therapy for refractory polymyositis and dermatomyositis. J Rheumatol 33: 1021–1026
Levine TD (2005) Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum 52: 601–607
Wylam ME et al. (2003) Successful treatment of refractory myasthenia gravis using rituximab: a pediatric case report. J Pediatr 143: 674–677
Illa I et al. (2008) Rituximab in refractory myasthenia gravis: a follow-up study of patients with anti-AChR or anti-MuSK antibodies [abstract #P06.001]. Neurology 70 (Suppl 1): A301
Tandam R et al. (2008) Pilot trial of rituximab in myasthenia gravis [abstract #P06.002]. Neurology 70 (Suppl 1): A301
Frenay CL et al. (2008) Rituximab for treatment of refractory myasthenia gravis [abstract #S57.003]. Neurology 70 (Suppl 1): A427
Leandro MJ et al. (2006) Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 54: 613–620
Dorner T (2006) Crossroads of B cell activation in autoimmunity: rationale of targeting B cells. J Rheumatol Suppl 77: 3–11
Pescovitz MD (2006) Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. Am J Transplant 6: 859–866.
Amanna IJ et al. (2007) Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 357: 1903–1915
Keogh KA et al. (2005) Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 52: 262–268
Kleinschmidt-DeMasters BK and Tyler KL (2005) Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 353: 369–374
FDA Public Health Advisory (2006) Life-threatening brain infection in patients with systemic lupus erythematosus after Rituxan (rituximab) treatment. [http://www.fda.gov/cder/drug/advisory/rituximab.htm]
Acknowledgements
The author thanks Dr R Raju for performing the B-cell counts in his patients treated with rituximab.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Dalakas, M. B cells as therapeutic targets in autoimmune neurological disorders. Nat Rev Neurol 4, 557–567 (2008). https://doi.org/10.1038/ncpneuro0901
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0901
This article is cited by
-
Monoclonal Antibody Therapies Beyond Complement for NMOSD and MOGAD
Neurotherapeutics (2022)
-
Stiff-person Syndrome and GAD Antibody-spectrum Disorders: GABAergic Neuronal Excitability, Immunopathogenesis and Update on Antibody Therapies
Neurotherapeutics (2022)
-
Autoimmune Neurological Disorders with IgG4 Antibodies: a Distinct Disease Spectrum with Unique IgG4 Functions Responding to Anti-B Cell Therapies
Neurotherapeutics (2022)
-
Polymyositis and dermatomyositis: ocular manifestations and potential sight-threatening complications
Rheumatology International (2022)
-
Evolution of Anti-B Cell Therapeutics in Autoimmune Neurological Diseases
Neurotherapeutics (2022)