Abstract
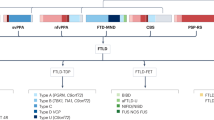
New findings relating to the clinical, genetic and molecular bases of neurodegenerative disorders have led to a shift away from traditional nomenclatures of clinical syndromes. Historically, frontotemporal lobar degeneration (FTLD), corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) were classified on the basis of distinct clinical and pathological features. In recent years, however, advances in molecular and genetic research have led clinicians to suggest that the similar etiologies of the three disorders warrant their amalgamation into a single disorder with three subtypes. In this Review, we consider the utility and validity of combining FTLD, CBD and PSP. The earliest reports of these disorders demonstrate their distinctiveness, whereas recent findings challenge traditional nomenclatures by showing etiological overlap. For example, tau inclusions have been confirmed in patients with CBD and those with PSP, and in some patients with FTLD, implying that all three disorders are 'tauopathies'. Furthermore, most patients with progressive nonfluent aphasia, a subtype of FTLD, show PSP or CBD post-mortem. Even tau-related cases of FTLD, CBD and PSP are distinguishable on the basis of other criteria, however, and many FTLD cases do not show tau pathology. We argue, therefore, that FTLD, CBD and PSP should be considered as pathologically similar but distinct syndromes. New research criteria for CBD and PSP should note that progressive nonfluent aphasia is often a precursor of these conditions.
Key Points
-
Traditionally, frontotemporal lobar degeneration (FTLD), progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) have been distinguishable on the basis of presenting clinical symptoms
-
Recently, molecular and genetic findings have suggested a link between the three disorders
-
PSP and CBD cases show tau inclusions post-mortem, as do some FTLD cases
-
Progressive nonfluent aphasia, a subtype of FTLD, often leads to PSP and CBD
-
Not all FTLD cases are tauopathies
-
As there are currently no treatments available that target the molecular and genetic etiologies of these three disorders, they should continue to be considered distinct
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kertesz A et al. (2003) Frontotemporal degeneration, Pick's disease, Pick complex, and Ravel. Ann Neurol 54 (Suppl 5): S1–S2
Kertesz A (2006) Progress in clinical neurosciences: frontotemporal dementia–Pick's disease. Can J Neurol Sci 33: 141–148
Josephs KA et al. (2006) Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 66: 41–48
Rebeiz JJ et al. (1968) Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 18: 20–33
Gibb WRG et al. (1989) Corticobasal degeneration. Brain 112: 1171–1192
Leiguarda R et al. (1994) The nature of apraxia in corticobasal degeneration. J Neurol Neurosurg Psychiatry 57: 455–459
Kertesz A et al. (2000) The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 55: 1368–1375
Leiguarda RC et al. (2003) Limb-kinetic apraxia in corticobasal degeneration: clinical and kinematic features. Mov Disord 18: 49–59
Grimes DA et al. (1999) Dementia as the most common presentation of cortical–basal ganglionic degeneration. Neurology 53: 1969–1974
Pillon B et al. (1995) The neuropsychological pattern of corticobasal degeneration: comparison with progressive supranuclear palsy and Alzheimer's disease. Neurology 45: 1477–1483
Dubois B et al. (2000) The FAB: a frontal assessment battery at bedside. Neurology 55: 1621–1626
Frattali CM et al. (2000) Language disturbances in corticobasal degeneration. Neurology 54: 990–992
Graham NL et al. (2003) Corticobasal degeneration as a cognitive disorder. Mov Disord 18: 1224–1232
Dickson DW et al. (2002) Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61: 935–946
Bugiani O et al. (1999) Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol 58: 667–677
Morris HR et al. (1999) The tau gene A0 polymorphism in progressive supranuclear palsy and related neurodegenerative diseases. J Neurol Neurosurg Psychiatry 66: 665–667
Steele JC et al. (1964) Progressive supranuclear palsy: a heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10: 333–359
Troost BT and Daroff RB (1977) The ocular motor defects in progressive supranuclear palsy. Ann Neurol 2: 397–403
Rivaud-Pechoux S et al. (2000) Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology 54: 1029–1032
Maher ER and Lees AJ (1986) The clinical features and natural history of the Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 36: 1005–1008
Litvan I et al. (1996) Natural history of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 60: 615–620
Santacruz P et al. (1998) Progressive supranuclear palsy: a survey of the disease course. Neurology 50: 1637–1647
Litvan I et al. (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47: 1–9
Belfor N et al. (2006) Clinical and neuropsychological features of corticobasal degeneration. Mech Ageing Dev 127: 203–207
Davis PH et al. (1985) Atypical presentation of progressive supranuclear palsy. Ann Neurol 17: 337–343
Gearing M et al. (1994) Progressive supranuclear palsy: neuropathologic and clinical heterogeneity. Neurology 44: 1015–1024
Albert ML et al. (1974) The 'subcortical dementia' of progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 37: 121–130
Maher ER et al. (1985) Cognitive deficits in the Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). J Neurol Neurosurg Psychiatry 48: 1234–1239
Grafman J et al. (1990) Frontal lobe function in progressive supranuclear palsy. Arch Neurol 47: 553–558
Hauw JJ et al. (1994) Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 44: 2015–2019
Wszolek ZK et al. (1998) Clinical neurophysiologic findings in patients with rapidly progressive familial parkinsonism and dementia with pallido-ponto-nigral degeneration. Electroencephalogr Clin Neurophysiol 107: 213–222
Conrad C et al. (1997) Genetic evidence for the involvement of tau in progressive supranuclear palsy. Ann Neurol 41: 277–281
Baker M et al. (1999) Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 8: 711–715
Flament S et al. (1991) Abnormal tau proteins in progressive supranuclear palsy: similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol (Berl) 81: 591–596
Gotz J et al. (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Aβ42 fibrils. Science 293: 1491–1495
Roy S et al. (1974) Electron microscopic study of neurofibrillary tangles in Steele–Richardson–Olszewski syndrome. Acta Neuropathol (Berl) 29: 175–179
Pick A (1892) On the relationship between senile brain atrophy and aphasia [German]. Prager Medizinische Wochenschrift 17: 165–167
Alzheimer AC (1911) On peculiar cases of disease at higher age [German]. Zeitschrift für die gesamte Neurologie und Psychiatrie 4: 356–385
Onari K and Spatz H (1926) Anatomical contributions on Pick's circumscribed cerebral cortex atrophy (Pick's disease) [German]. Zeitschrift für die gesamte Neurologie und Psychiatrie 101: 470–511
[No authors listed] (1994) Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester groups. J Neurol Neurosurg Psychiatry 57: 416–418
Neary D et al. (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51: 1546–1554
Mott RT et al. (2005) Neuropathologic, biochemical, and molecular characterization of the frontotemporal dementias. J Neuropathol Exp Neurol 64: 420–428
McKhann GM et al. (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol 58: 1803–1809
Foster NL et al. (1997) Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference Participants. Ann Neurol 41: 706–715
Wilhelmsen KC et al. (1994) Localization of disinhibition–dementia–parkinsonism–amyotrophy complex to 17q21-22. Am J Hum Genet 55: 1159–1165
Baker M et al. (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442: 916–919
Morita M et al. (2006) A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66: 839–844
Hughes A et al. (2003) Tau haplotype frequency in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Exp Neurol 181: 12–16
Panegyres PK and Zafiris-Toufexis K (2002) Polymorphisms in the tau gene in sporadic frontotemporal dementia and other neurodegenerative disorders. Eur J Neurol 9: 485–489
Gorno-Tempini ML et al. (2004) Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase 10: 426–436
Houlden H et al. (2001) Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 56: 1702–1706
Di Maria E et al. (2000) Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann Neurol 47: 374–377
Feany MB and Dickson DW (1996) Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol 40: 139–148
Soliveri P et al. (2003) A case of dementia parkinsonism resembling progressive supranuclear palsy due to mutation in the tau protein gene. Arch Neurol 60: 1454–1456
Tsuboi Y et al. (2005) Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov Disord 20: 982–988
Kertesz A et al. (2005) The evolution and pathology of frontotemporal dementia. Brain 128: 1996–2005
Hodges JR et al. (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56: 399–406
Neary D (1997) Frontotemporal degeneration, Pick disease, and corticobasal degeneration. One entity or 3? 3. Arch Neurol 54: 1425–1427
Boeve BF et al. (2003) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54 (Suppl 5): S15–S19
Noble W et al. (2005) Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA 102: 6990–6995
Acknowledgements
This work was supported by the Larry L Hillblom Foundation, National Institute on Aging grant P01-AG1972403, and an Alzheimer's Disease Research Center grant P50-AG023501.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sha, S., Hou, C., Viskontas, I. et al. Are frontotemporal lobar degeneration, progressive supranuclear palsy and corticobasal degeneration distinct diseases?. Nat Rev Neurol 2, 658–665 (2006). https://doi.org/10.1038/ncpneuro0357
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0357
This article is cited by
-
Dementia Risk in Posttraumatic Stress Disorder: the Relevance of Sleep-Related Abnormalities in Brain Structure, Amyloid, and Inflammation
Current Psychiatry Reports (2017)
-
Ocular motor abnormalities in neurodegenerative disorders
Eye (2015)
-
Treatment Options for Tauopathies
Current Treatment Options in Neurology (2012)
-
Extrapyramidal Syndromes in Frontotemporal Degeneration
Journal of Molecular Neuroscience (2011)