Abstract
Diabetic neuropathy (DN) is the most common complication of diabetes mellitus, and it imposes a considerable burden on a patient's quality of life and the health-care system. Despite the prevalence and severity of DN, there are no effective treatments. Pathogenetic evidence suggests that DN is marked by degeneration of dorsal root ganglion (DRG) neurons in peripheral nerves, and that DRG mitochondria are particularly affected. DRG mitochondria are especially vulnerable because they are the origin of reactive oxygen species production in the hyperglycemic neuron. Accumulating evidence indicates that neuronal mitochondria are subject to damage at the level of their DNA, and their outer and inner membranes, and also via deregulation of mitochondrial fission and fusion proteins that control mitochondrial shape and number. This Review will survey the mechanisms of mitochondrial degeneration in the pathogenesis of DN, highlighting potential mitochondrial sites for therapeutic intervention.
Key Points
-
Diabetic neuropathy (DN) results from hyperglycemia-induced damage to the peripheral nervous system, as indicated by loss of Schwann cells, myelinated axons, and a population of sensory neurons located in the dorsal root ganglia
-
Hyperglycemia increases the production and stabilization of reactive oxygen species (ROS) that damage peripheral neurons, particularly at the level of mitochondria
-
ROS might compromise the integrity of the mitochondrial genome, thereby contributing to the mitochondrial dysfunction underlying DN
-
ROS induce local apoptosis in peripheral neurons via actions of caspases and proapoptotic Bcl proteins that damage mitochondria, and ultimately impair neuronal function and viability
-
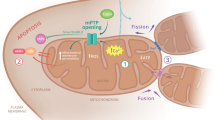
As recently shown for several hereditary neuropathies, dysregulated mitochondrial fission and fusion might have a pathogenetic role in mitochondrial damage that underlies the neuronal degeneration and functional loss in DN
-
Therapies aimed at preserving mitochondrial structure and function could be beneficial treatments for DN
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mokdad AH et al. (2001) The continuing epidemics of obesity and diabetes in the United States. JAMA 286: 1195–1200
Sheetz MJ and King GL (2002) Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA 288: 2579–2588
Feldman EL et al. (1999) Diabetic neuropathy. In Current Review of Diabetes, 71–83 (Ed. Taylor S) Philadelphia: Current Medicine
Vileikyte L (2001) Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev 17: 246–249
Ramsey SD et al. (1999) Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 22: 382–387
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986
[No authors listed] (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853
Kalichman MW et al. (1998) Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol (Berl) 95: 47–56
Chopra JS et al. (1977) Pathology and time relationship of peripheral nerve changes in experimental diabetes. J Neurol Sci 32: 53–67
Sendtner M et al. (1991) Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival. J Cell Sci Suppl 15: 103–109
Greene DA et al. (1992) Complications: neuropathy, pathogenetic considerations. Diabetes Care 15: 1902–1925
Schmeichel AM et al. (2003) Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes 52: 165–171
Sima AAF et al. (1988) Histopathological heterogeneity of neuropathy in insulin-dependent and non-insulin-dependent diabetes, and demonstrations of axo-glial dysjunction in human diabetic neuropathy. J Clin Invest 81: 349–364
Llewelyn JG et al. (1991) Sural nerve morphometry in diabetic autonomic and painful sensory neuropathy. Brain 114: 867–892
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820
Stevens MJ et al. (2002) Pathogenesis of diabetic neuropathy. In Ellenberg and Rifkin's Diabetes Mellitus, 747–770 (Eds Porte D Jr et al.) New York: McGraw Hill
Cameron NE et al. (2001) Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 44: 1973–1988
Bach D et al. (2005) Expression of Mfn2, the Charcot–Marie–Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor [alpha] and interleukin-6. Diabetes 54: 2685–2693
Bach D et al. (2003) Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism: a novel regulatory mechanism altered in obesity. J Biol Chem 278: 17190–17197
Russell JW et al. (2002) High glucose induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J 16: 1738–1748
Vincent AM et al. (2005) Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J 19: 638–640
Vincent AM et al. (2005) Cell culture modeling to test therapies against hyperglycemia-mediated oxidative stress and injury. Antiox Redox Signal 7: 1494–1506
Leinninger GM et al. (2004) Insulin-like growth factor-I (IGF-I) regulates glucose-induced mitochondrial depolarization and apoptosis in human neuroblastoma. Cell Death Differ 11: 885–896
Leinninger GM et al. (2004) Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J 18: 1544–1546
Kim JY et al. (2005) Mitochondrial DNA content is decreased in autosomal dominant optic atrophy. Neurology 64: 966–972
Harrison JF et al. (2005) Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res 33: 4660–4671
Ryu H et al. (2005) Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc Natl Acad Sci USA 102: 13915–13920
Mehrabian Z et al. (2005) Regulation of mitochondrial gene expression by energy demand in neural cells. J Neurochem 93: 850–860
Peltier AC and Russell JW (2002) Recent advances in drug-induced neuropathies. Curr Opin Neurol 15: 633–638
Raff MC et al. (2002) Axonal self-destruction and neurodegeneration. Science 296: 868–871
Greene DA et al. (1999) Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur J Pharmacol 375: 217–223
Russell JW et al. (1999) Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis 6: 347–363
Cheng C and Zochodne DW (2003) Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes 52: 2363–2371
Srinivasan S et al. (2000) Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes 49: 1932–1938
Zochodne DW et al. (2001) Does diabetes target ganglion neurones? Progressive sensory neurone involvement in long-term experimental diabetes. Brain 124: 2319–2334
Raff MC et al. (2002) Axonal self-destruction and neurodegeneration. Science 296: 868–871
Cellerino A et al. (2000) Apoptosis in the developing visual system. Cell Tissue Res 301: 53–69
Graham SH et al. (2000) Bcl-2 family gene products in cerebral ischemia and traumatic brain injury. J Neurotrauma 17: 831–841
Yamaguchi H and Wang HG (2002) Bcl-XL protects BimEL-induced Bax conformational change and cytochrome C release independent of interacting with Bax or BimEL. J Biol Chem 277: 41604–41612
Eskes R et al. (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20: 929–935
Marani M et al. (2002) Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol 22: 3577–3589
Uo T et al. (2004) Neurons exclusively express N-BAK, a BH3 domain-only BAK isoform that promotes neuronal apoptosis. J Biol Chem 250: 9065–9073
Degli EM and Dive C (2003) Mitochondrial membrane permeabilisation by Bax/Bak. Biochem Biophys Res Commun 304: 455–461
Makin GW et al. (2001) Damage-induced Bax N-terminal change, translocation to mitochondria and formation of Bax dimers/complexes occur regardless of cell fate. EMBO J 20: 6306–6315
Antonsson B et al. (2000) Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J 345 Pt 2: 271–278
Kuwana T et al. (2002) Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111: 331–342
Ahmad KA et al. (2004) Hydrogen peroxide-mediated cytosolic acidification is a signal for mitochondrial translocation of Bax during drug-induced apoptosis of tumor cells. Cancer Res 64: 7867–7878
Perier C et al. (2005) Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci USA 102: 19126–19131
Leinninger GM et al. (2006) Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis 23: 11–22
Rube DA and van der Bliek AM (2004) Mitochondrial morphology is dynamic and varied. Mol Cell Biochem 256–257: 331–339
Karbowski M and Youle RJ (2003) Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ 10: 870–880
Szabadkai G et al. (2004) Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2 waves and protects against Ca2-mediated apoptosis. Mol Cell 16: 59–68
Karbowski M et al. (2004) Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol 164: 493–499
Züchner S et al. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot–Marie–Tooth neuropathy type 2A. Nat Genet 36: 449–451
Delettre C et al. (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26: 207–210
Lee YJ et al. (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011
Karbowski M et al. (2002) Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159: 931–938
Arai R et al. (2004) Establishment of stable hFis1 knockdown cells with an siRNA expression vector. J Biochem (Tokyo) 136: 421–425
Frank S et al. (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525
Yu T et al. (2006) Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103: 2653–2658
Sundkvist G et al. (2000) Sorbitol and myo-inositol levels and morphology of sural nerve in relation to peripheral nerve function and clinical neuropathy in men with diabetic, impaired, and normal glucose tolerance. Diabet Med 17: 259–268
Rudofsky G Jr et al. (2006) Functional polymorphisms of UCP2 and UCP3 are associated with a reduced prevalence of diabetic neuropathy in patients with type 1 diabetes. Diabetes Care 29: 89–94
Brussee V et al. (2004) Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes 53: 1824–1830
Huang TJ et al. (2005) Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol 194: 279–283
Huang TJ et al. (2003) Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes 52: 2129–2136
Ilnytska O et al. (2006) Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes 55: 1686–1694
Bouchier-Hayes L et al. (2005) Mitochondria: pharmacological manipulation of cell death. J Clin Invest 115: 2640–2647
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Leinninger, G., Edwards, J., Lipshaw, M. et al. Mechanisms of Disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat Rev Neurol 2, 620–628 (2006). https://doi.org/10.1038/ncpneuro0320
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0320
This article is cited by
-
The therapeutic effect of Salvia spinosa on diabetic neuropathy induced by STZ via attenuation of the oxidative pathway
Comparative Clinical Pathology (2022)
-
Fisetin Imparts Neuroprotection in Experimental Diabetic Neuropathy by Modulating Nrf2 and NF-κB Pathways
Cellular and Molecular Neurobiology (2016)
-
Anti-inflammatory effect of AMPK signaling pathway in rat model of diabetic neuropathy
Inflammopharmacology (2016)
-
New Insights in (Inter)Cellular Mechanisms by Heart Failure with Preserved Ejection Fraction
Current Heart Failure Reports (2014)
-
Protective Effects of Salvianolic Acid B on Schwann Cells Apoptosis Induced by High Glucose
Neurochemical Research (2012)