Abstract
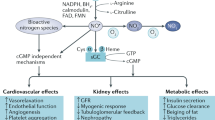
Nitric oxide (NO) production is reduced in renal disease, partially due to decreased endothelial NO production. Evidence indicates that NO deficiency contributes to cardiovascular events and progression of kidney damage. Two possible causes of NO deficiency are substrate (L-arginine) limitation and increased levels of circulating endogenous inhibitors of NO synthase (particularly asymmetric dimethylarginine [ADMA]). Decreased L-arginine availability in chronic kidney disease (CKD) is due to perturbed renal biosynthesis of this amino acid. In addition, inhibition of transport of L-arginine into endothelial cells and shunting of L-arginine into other metabolic pathways (e.g. those involving arginase) might also decrease availability. Elevated plasma and tissue levels of ADMA in CKD are functions of both reduced renal excretion and reduced catabolism by dimethylarginine dimethylaminohydrolase (DDAH). The latter might be associated with loss-of-function polymorphisms of a DDAH gene, functional inhibition of the enzyme by oxidative stress in CKD and end-stage renal disease, or both. These findings provide the rationale for novel therapies, including supplementation of dietary L-arginine or its precursor L-citrulline, inhibition of non-NO-producing pathways of L-arginine utilization, or both. Because an increase in ADMA has emerged as a major independent risk factor in end-stage renal disease (and probably also in CKD), lowering ADMA concentration is a major therapeutic goal; interventions that enhance the activity of the ADMA-hydrolyzing enzyme DDAH are under investigation.
Key Points
-
Production of nitric oxide (NO; from L-arginine and O2 via nitric oxide synthase [NOS]) is reduced in renal disease
-
As chronic NOS inhibition produces hypertension and renal dysfunction in animals, NO deficiency probably contributes to progression of kidney disease in humans
-
Net NO deficiency can develop in response to decreased substrate availability (e.g. perturbed synthesis or transport of arginine) and the action of endogenous NOS inhibitors (e.g. asymmetric dimethylarginine)
-
Potential therapies for kidney disease include supplementation of dietary L-arginine, inhibition of non-NO-producing biochemical processes that consume L-arginine, and decreasing the activity of asymmetric dimethylarginine
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Baylis C and Vallance P (1998) Measurement of nitrite and nitrate (NOx) levels in plasma and urine; what does this measure tell us about the activity of the endogenous nitric oxide. Current Opinion Nephrol Hypertens 7: 1–4
Schmidt R et al. (1999) Indices of activity of the nitric oxide system in patients on hemodialysis. Am J Kidney Dis 34: 228–234
Schmidt RJ et al. (1999) Nitric oxide production is low in end stage renal disease patients on peritoneal dialysis. Am J Physiol 276: F794–F797
Schmidt RJ and Baylis C (2000) Total nitric oxide production is low in patients with chronic renal disease. Kidney Int 58: 1261–1266
Blum M et al. (1998) Low nitric oxide production in patients with chronic renal failure. Nephron 79: 265–268
Wever R et al. (1999) Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 19: 1168–1172
Lau T et al. (2000) Arginine, citrulline, and nitric oxide metabolism in end-stage renal disease patients. J Clin Invest 105: 1217–1225
Vaziri ND et al. (1998) Downregulation of nitric oxide synthase in chronic renal insufficiency: role of excess PTH. Am J Physiol 274: F642–F649
Wagner L et al. (2002) Reduced NOS activity in rats with chronic renal disease due to glomerulonephritis. Kidney Int 62: 532–536
Erdely A et al. (2003) Protection of Wistar Furth rats from chronic renal disease is associated with maintained renal nitric oxide synthase. J Am Soc Nephrol 14: 2526–2533
Szabo A et al. (2003) Renal neuronal nitric oxide synthase protein expression as a marker of renal function. Kidney Int 64: 1765–1771
Erdely A et al. (2004) Protection against puromycin aminonucleoside-induced chronic renal disease in the Wistar-Furth rat. Am J Physiol 287: F81–F89
Erdely A et al. (2003) Sexual dimorphism in the aging kidney: effects on injury and nitric oxide system. Kidney Int 63: 1021–1026
Thambyrajah J et al. (2000) Abnormalities of endothelial function in patients with predialysis renal failure. Heart 83: 205–209
Böger RH and Zoccali C (2003) ADMA: a novel risk factor that explains excess cardiovascular event rate in patients with end-stage renal disease. Atherosclerosis (Suppl 4): S23–S28
Foley RN et al. (1998) Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32 (Suppl 3): S112–S119
Baigent C et al. (2000) Premature cardiovascular disease in chronic renal failure. Lancet 356: 147–152
Landray MJ et al. (2004) Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis 43: 244–253
Himmelfarb J (2004) Linking oxidative stress and inflammation in kidney disease: which is the chicken and which is the egg? Semin Dial 17: 449–454
Zatz R and Baylis C (1998) Chronic nitric oxide inhibition model six years on. Hypertension 32: 958–964
Peppa M et al. (2004) Glycoxidation and inflammation in renal failure patients. Am J Kidney Dis 43: 690–695
Wu G and Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 33: 1–17
Brosnan ME and Brosnan JT (2004) Renal arginine metabolism. J Nutr 134 (Suppl 10): S2791–S2795
Tizianello A et al. (1980) Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest 65: 1162–1173
Reyes AA et al. (1994) Role of arginine in health and in renal disease. Am J Physiol 267: F331–F346
Xiao S et al. (2001) Uremic levels of urea inhibit L-arginine transport in cultured endothelial cells. Am J Physiol Renal Physiol 280: F989–F995
Wagner L et al. (2002) Urea transporters are widely distributed in endothelial cells and mediate inhibition of L-arginine transport. Am J Physiol Renal Physiol 283: F578–F582
Devés R and Boyd CAR (1998) Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev 78: 487–545
Swendseid ME et al. (1975) Amino acid metabolism in the chronically uremic rat. Clin Nephrol 3: 240–246
Bergström J et al. (1990) Plasma and muscle free amino acids in maintenance hemodialysis patients without protein malnutrition. Kidney Int 38: 108–114
Vallance P et al. (1992) Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575
Fleck C et al. (2001) Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in renal failure patients. Kidney Int Suppl 78: S14–S18
Kielstein JT et al. (1999) Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol 10: 594–600
Wahbi N et al. (2001) Dimethylarginines in chronic renal failure. J Clin Pathol 54: 470–473
Bogle RG et al. (1995) Induction of NG-monomethyl-L-arginine uptake: a mechanism for differential inhibition of NO synthases? Am J Physiol Cell Physiol 269: C750–C756
Closs EI et al. (1997) Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier HCAT-2B. Nitric Oxide 1: 65–73
Schwartz IF et al. (2006) Arginine uptake is attenuated, through modulation of cationic amino acid transporter-1, in uremic rats. Kidney Int 69: 298–303
Mendes Ribeiro AC et al. (1999) Identification of system y+L as the high-affinity transporter for L-arginine in human platelets: up-regulation of L-arginine influx in uraemia. Eur J Physiol 438: 573–575
Mendes Ribeiro AC et al. (1997) Transport of L-arginine and the nitric oxide inhibitor NG-monomethyl-L-arginine in human erythrocytes in chronic renal failure. Clin Sci 93: 57–64
Cross JM et al. (2001) Acute administration of L-arginine does not improve arterial endothelial function in chronic renal failure. Kidney Int 60: 2318–2323
Bennett–Richards KJ et al. (2002) Oral L-arginine does not improve endothelial dysfunction in children with chronic renal failure. Kidney Int 62: 1372–1378
Hand MF et al. (1998) Hemodialysis and L-arginine, but not D-arginine, correct renal failure-associated endothelial dysfunction. Kidney Int 53: 1068–1077
De Nicola L et al. (1999) Randomized, double-blind, placebo-controlled study of arginine supplementation in chronic renal failure. Kidney Int 56: 674–684
Zhang XZ et al. (2001) L-arginine supplementation in young renal allograft recipients with chronic transplant dysfunction. Clin Nephrol 55: 453–459
Peters H et al. (1999) From rats to man: a perspective on dietary L-arginine supplementation in human renal disease. Nephrol Dial Transplant 14: 1640–1650
McDonald KK et al. (1997) A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem 272: 31213–31216
Kurtz S and Harrison DG (1997) Insulin and the arginine paradox. J Clin Invest 99: 369–370
Palmer RMJ et al. (1988) Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 333: 664–666
Li C et al. (2005) Interaction of the eNOS with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport. Biochem J 386: 567–574
Segal MS et al. (2006) Endothelial health and diversity in the kidney. J Am Soc Nephrol 7: 323–324
Zani BG and Bohlen HG (2005) Transport of extracellular L-arginine via cationic amino acid transporter is required during in vivo endothelial NO production. Am J Physiol Heart Circ Physiol 289: H1381–H1390
Ishizuka S et al. (2000) Agmatine inhibits cell proliferation and improves renal function in anti-Thy-1 glomerulonephritis. J Am Soc Nephrol 11: 2256–2264
Schwartz D et al. (1997) Agmatine affects glomerular filtration via a nitric oxide synthase-dependent mechanism. Am J Physiol 272: F597–F601
Morrissey JJ and Klahr S (1997) Agmatine activation of nitric oxide synthase in endothelial cells. Proc Assoc Am Physicians 109: 51–57
Li H et al. (2001) Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 280: E75–E82
Sabbatini M et al. (2003) Arginase inhibition slows the progression of renal failure in rats and renal ablation. Am J Physiol Renal Physiol 284: F680–F687
Zhang C et al. (2004) Upregulation of vascular arginase in hypertension decreases nitric oxide mediated dilation of coronary arterioles. Hypertension 44: 935–943
Johnson FK et al. (2005) Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol 288: 1057–1062
Demougeot C et al. (2005) Arginase inhibition reduces endothelial dysfunction and blood pressure rising in SHR. J Hypertension 23: 971–978
Xiao S et al. (2000) Plasma from ESRD patients inhibits nitric oxide synthase (NOS) activity in cultured human and bovine endothelial cells. Acta Phys Scand 168: 175–179
McAllister RJ et al. (1994) Effects of guanidino and uremic compounds on nitric oxide pathways. Kidney Int 45: 737–742
Leiper J and Vallance P (1999) Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 43: 542–548
Achan V et al. (2003) Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23: 1455–1459
Kielstein JT et al. (2004) Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetric dimethylarginine in humans. Circulation 109: 172–177
Vallance P and Leiper J (2005) ADMA and kidney disease—marker or mediator. J Am Soc Nephrol 16: 2254–2256
Scalera F et al. (2005) Erythropoietin increases asymmetric dimethylarginine in endothelial cells: role of dimethylarginine dimethylaminohydrolase. J Am Soc Nephrol 16: 892–898
Ueno S et al. (1992) Distribution of free methylarginines in rat tissues and in the bovine brain. J Neurochem 59: 2012–2016
Casellas P and Jeanteur P (1978) Protein methylation in animal cells. I. Purification and properties of S-adenosyl-L-methionine: protein (arginine) N-methyltransferase from Krebs II ascites cells. Biochim Biophys Acta 519: 243–254
Casellas P and Jeanteur P (1978) Protein methylation in animal cells. II. Inhibition of S-adenosyl–L–methionine: protein (arginine) N–methyltransferase by analogs of S-adenosyl-L-homocysteine. Biochim Biophys Acta 519: 255–268
Liu Q and Dreyfuss G (1995) In vivo and in vitro arginine methylation of RNA–binding proteins. Mol Cell Biol 15: 2800–2808
Böger RH et al. (2000) LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 87: 99–105
Osanai T et al. (2003) Effect of shear stress on asymmetric dimethylarginine release from vascular endothelial cells. Hypertension 42: 985–990
Leiper JM et al. (1999) Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 343: 209–214
Tojo A et al. (1997) Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int 52: 1593–1601
Kimoto M et al. (1993) Detection of NG, NG-dimethylarginine dimethylaminohydrolase in the nitric oxide-generating systems of rats using monoclonal antibody. Arch Biochem Biophys 300: 657–662
Nijveldt RJ et al. (2003) Handling of asymmetrical dimethylarginine and symmetrical dimethylarginine by the rat kidney under basal conditions and during endotoxaemia. Nephrol Dial Transplant 18: 2542–2550
Nijveldt RJ et al. (2002) Net renal extraction of asymmetrical (ADMA) and symmetrical (SDMA) dimethylarginine in fasting humans. Nephrol Dial Transplant 17: 1999–2002
MacAllister RJ et al. (1996) Regulation of nitric oxide synthesis by dimethlarginine dimethylaminohydrolase. British J Pharm 119: 1533–1540
Dayoub H et al. (2003) Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation 108: 3042–3047
Tran CT et al. (2000) Chromosomal localization, gene structure, and expression pattern of DDAH1: comparison with DDAH2 and implications for evolutionary origins. Genomics 68:101–105
Jones LC et al. (2003) Common genetic variation in a basal promoter element alters DDAH2 expression in endothelial cells. Biochem Biophys Res Commun 310: 836–843
Akbar F et al. (2005) Haplotypic association of DDAH1 with susceptibility to pre-eclampsia. Mol Hum Reprod 11: 73–77
Pettersson A et al. (1998) Increased circulating concentrations of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet Gynecol Scand 77: 808–813
Ito A et al. (1999) Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 99: 3092–3095
Leiper J et al. (2002) S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci USA 99: 13527–13532
Millatt LJ et al. (2003) Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation 108: 1493–1498
Lin KY et al. (2002) Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 106: 987–992
Stuhlinger MC et al. (2001) Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation 104: 2569–2575
Kimoto M et al. (1995) Probucol preserves endothelial function by reduction of the endogenous nitric oxide synthase inhibitor level. Br J Pharmacol 135: 1175–1182
Saran R et al. (2003) Impact of vitamin E on plasma asymmetric dimethylarginine (ADMA) in chronic kidney disease (CKD): a pilot study. Nephrol Dial Transplant 18: 2415–2420
Achan V et al. (2002) All-trans-retinoic acid increases nitric oxide synthesis by endothelial cells: a role for the induction of dimethylarginine dimethylaminohydrolase. Circ Res 90: 764–769
Deng S et al. (2004) Aspirin protected against endothelial damage induced by LDL: role of endogenous NO synthase inhibitors in rats. Acta Pharmacol Sin 25: 1633–1639
DP Holden et al. (2003) Estrogen stimulates dimethylarginine dimethylaminohydrolase activity and the metabolism of asymmetric dimethylarginine. Circulation 108: 1575–1580
Xiao S et al. (2001) Circulating eNOS inhibitory factor in some patients with chronic renal disease. Kidney Int 59: 1466–1472
Kielstein JT et al. (2002) Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol 13: 170–176
Zoccali C et al. (2001) Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 358: 2113–2117
Zoccali C et al. (2002) Asymmetric dimethylarginine, C–reactive protein, and carotid intima–media thickness in end-stage renal disease. J Am Soc Nephrol 13: 490–496
Zoccali C et al. (2002) Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int 62: 339–345
Surdacki A et al. (1999) Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol 33: 652–658
Valkonen VP et al. (2001) Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet 358: 2127–2128
Sydow K et al. (2004) Endothelial dysfunction in patients with peripheral arterial disease and chronic hyperhomocysteinemia: potential role of ADMA. Vasc Med 9: 93–101
Bode-Böger SM et al. (2005) Asymmetric dimethylarginine (ADMA) accelerates cell senescence. Vasc Med 10 (Suppl 1): S65–S71
Guthrie SM et al. (2004) The NO pathway modulates hemangioblast activity of adult hematopoietic stem cells. Blood 105: 1916–1922
Thum T et al. (2005) Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol 46: 1693–1701
Smith CL et al. (2003) Dimethylarginine dimethylaminohydrolase activity modulates ADMA levels, VEGF expression, and cell phenotype. Biochem Biophys Res Commun 308: 984–989
Azuma H et al. (1995) Accumulation of endogenous inhibitors for nitric oxide synthesis and decreased content of L-arginine in regenerated endothelial cells. Br J Pharmacol 115: 1001–1004
De Groot K et al. (2004) Uremia causes endothelial progenitor cell deficiency. Kidney Int 66: 641–646
Ravani P et al. (2005) Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol 16: 2449–2455
Fliser D et al. (2005) Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol 16: 2456–2461
Anderstam B et al. (1997) Serum levels of NG, NG-dimethyl-L-arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol 18: 1437–1442
Bergamini S et al. (2004) Relationship of asymmetric dimethylarginine to haemodialysis hypotension. Nitric Oxide 11: 273–278.
Kielstein JT et al. (2004) Low dialysance of asymmetric dimethylarginine (ADMA)—in vivo and in vitro evidence of significant protein binding. Clin Nephrol 62: 295–300
Baylis C (2005) Changes in renal hemodynamics and structure in the aging kidney; sexual dimorphism and the nitric oxide system. Exp Gerontol 40: 271–27855
Acknowledgements
The support of NIH grants R01 DK56843 and DK 45517 is gratefully acknowledged.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Baylis, C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Rev Nephrol 2, 209–220 (2006). https://doi.org/10.1038/ncpneph0143
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0143
This article is cited by
-
Diabetes-induced male infertility: potential mechanisms and treatment options
Molecular Medicine (2024)
-
β-Aminoisobutyric acid supplementation attenuated salt-sensitive hypertension in Dahl salt-sensitive rats through prevention of insufficient fumarase
Amino Acids (2022)
-
Nitric oxide signalling in kidney regulation and cardiometabolic health
Nature Reviews Nephrology (2021)
-
Cardiorenal metabolic biomarkers link early life stress to risk of non-communicable diseases and adverse mental health outcomes
Scientific Reports (2020)
-
Resistance training improves sleep quality, redox balance and inflammatory profile in maintenance hemodialysis patients: a randomized controlled trial
Scientific Reports (2020)