Abstract
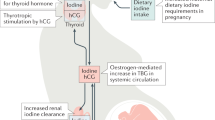
The transplacental passage of thyroid hormones from the maternal circulation to the fetal circulation within the human hemochorial placenta is important for normal fetal development, particularly the development of the central nervous system. The role of maternal thyroid hormones is particularly important in the first half of pregnancy, before the onset of endogenous thyroid hormone production in the fetus. The human placenta regulates the quantity and composition of different forms of transported thyroid hormones to ensure that the requisite levels are present in the fetus for each stage of development. Transplacental thyroid hormone supply to the fetus is modulated by several factors, including the following proteins: plasma membrane transporters, which regulate the passage of thyroid hormones in and out of cells; iodothyronine deiodinases, which metabolize thyroid hormones; and proteins within trophoblast cells, which bind thyroid hormones. In pathological situations of either maternal or fetal thyroid hormone deficiency during pregnancy, the placenta seems to lack the full compensatory mechanisms necessary to optimize maternal–fetal transfer of thyroid hormones. Inadequate passage of thyroid hormones can lead to suboptimal fetal thyroid hormone levels, which might contribute to the neurodevelopmental delay associated with such conditions. Thus, maintaining normal maternal thyroid hormone status is likely to be the primary factor in ensuring adequate transplacental thyroid hormone passage and appropriate iodide supply to the fetus.
Key Points
-
The transplacental passage of thyroid hormones from the maternal circulation to the fetal circulation is important from the first trimester of pregnancy to ensure normal fetal development, particularly of the central nervous system
-
In normal pregnancy, transplacental thyroid hormone passage is modulated by plasma membrane thyroid hormone transporters, the metabolism of thyroid hormones by iodothyronine deiodinases, and the binding of thyroid hormones to several different proteins within placental trophoblast cells
-
In pathological pregnancies with either maternal or fetal thyroid hormone deficiency, the placenta lacks the full compensatory mechanisms necessary to optimize maternal–fetal transfer of thyroid hormones to achieve normal thyroid hormone levels in the fetus
-
Maintaining normal maternal thyroid hormone status is likely to be the primary factor in determining adequate transplacental thyroid hormone passage and appropriate iodide supply to the fetus
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Haddow JE et al. (1999) Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341: 549−555
Pop VJ et al. (2003) Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 59: 282–288
de Escobar GM et al. (2004) Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18: 225–248
Chan S. et al. (2002) Early expression of thyroid hormone deiodinases and receptors in human fetal cerebral cortex. Brain Res Dev Brain Res 138: 109–116
Sinha AK et al. (1997) Thyroid hormone and brain maturation. In Recent Research Developments in Neuroendocrinology, 1–14 (Ed Hendrich CE). Trivandrum: India Research Signpost
Chan S et al. (2005) Maternal thyroid hormones and fetal brain development. Curr Opin Endocrinol Diabetes 12: 23–30
Kilby MD et al. (2005) Thyroid hormone action in the placenta. Placenta 26: 105–113
Barber KJ et al. (2005) The in vitro effects of triiodothyronine on epidermal growth factor-induced trophoblast function. J Clin Endocrinol Metab 90: 1655–1661
LaFranchi SH et al. (2005) Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid 15: 60–71
Casey BM et al. (2005) Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 105: 239–245
Negro R et al. (2007) Euthyroid women with autoimmune disease undergoing assisted reproduction technologies: the role of autoimmunity and thyroid function. J Endocrinol Invest 30: 3–8
Grumbach MM and Werner SC (1956) Transfer of thyroid hormone across the human placenta at term. J Clin Endocrinol Metab 16: 1392–1395
Vulsma T et al. (1989) Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med 321: 13–16
Calvo RM et al. (2002) Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab 87: 1768–1777
Costa A et al. (1991) Thyroid hormones in tissues from human embryos and fetuses. J Endocrinol Invest 14: 559–568
Mandel SJ et al. (2005) Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid 15: 44–53
Hume R et al. (2004) Human fetal and cord serum thyroid hormones: developmental trends and interrelationships. J Clin Endocrinol Metab 89: 4097–4103
Bianco AC et al. (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23: 38–89
Visser TJ (1996) Pathways of thyroid hormone metabolism. Acta Med Austriaca 23: 10–16
Ferreiro B et al. (1988) Estimation of nuclear thyroid hormone receptor saturation in human fetal brain and lung during early gestation. J Clin Endocrinol Metab 67: 853–856
Richard K et al. (1998) Ontogeny of iodothyronine deiodinases in human liver. J Clin Endocrinol Metab 83: 2868–2874
Farwell AP et al. (2005) Regulation of cerebellar neuronal migration and neurite outgrowth by thyroxine and 3,3′,5′-triiodothyronine. Brain Res Dev Brain Res 154: 121–135
Farwell AP et al. (2006) Dynamic nongenomic actions of thyroid hormone in the developing rat brain. Endocrinology 147: 2567–2574
Jauniaux E et al. (1991) In vivo investigations of the anatomy and the physiology of early human placental circulations. Ultrasound Obstet Gynecol 1: 435–445
Schneider H (1991) Placental Transport Function. Reprod Fertil Dev 3: 345–353
Chan S et al. (2003) Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J Clin Endocrinol Metab 88: 4488–4495
Huang SA et al. (2003) Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab 88: 1384–1388
Roti E et al. (1983) Inner ring deiodination of thyroxine and 3,5,3′-triiodothyronine by human fetal membranes. Am J Obstet Gynecol 147: 788–792
de Escobar GM et al. (1993) Effects of iodine deficiency on thyroid hormone metabolism and the brain in fetal rats: the role of the maternal transfer of thyroxin. Am J Clin Nutr 57 (Suppl 2): S280–S285
Dowling AL et al. (2000) Acute changes in maternal thyroid hormone induce rapid and transient changes in gene expression in fetal rat brain. J Neurosci 20: 2255–2265
Mitchell AM et al. (1992) Uptake of L-tri-iodothyronine by human cultured trophoblast cells. J Endocrinol 133: 483–486
Friesema ECH et al. (2005) Membrane transporters for thyroid hormone. Curr Opin Endocrinol Diabetes 12: 371–380
Friesema ECH et al. (2004) Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364: 1435–1437
Dumitrescu AM et al. (2004) A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175
Heuer H et al. (2005) The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology 146: 1701–1706
Chan SY et al. (2006) Monocarboxylate transporter 8 expression in the human placenta: the effects of severe intrauterine growth restriction. J Endocrinol 189: 465–471
Kim DK et al. (2001) Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. J Biol Chem 276: 17221–17228
Ritchie JW and Taylor PM (2001) Role of the System L permease LAT1 in amino acid and iodothyronine transport in placenta. Biochem J 356: 719–725
Park SY et al. (2005) Reabsorption of neutral amino acids mediated by amino acid transporter LAT2 and TAT1 in the basolateral membrane of proximal tubule. Arch Pharm Res 28: 421–432
Patel P et al. (2003) Semi quantitative expression analysis of MDR3, FIC1, BSEP, OATP-A, OATP-C,OATP-D, OATP-E and NTCP gene transcripts in 1st and 3rd trimester human placenta. Placenta 24: 39–44
Sato K et al. (2003) Expression of organic anion transporting polypeptide E (OATP-E) in human placenta. Placenta 24: 144–148
Ritchie JWA et al. (2003) A role for thyroid hormone transporters in transcriptional regulation by thyroid hormone receptors. Mol Endocrinol 17: 653–661
Koopdonk-Kool JM et al. (1996) Type II and type III deiodinase activity in human placenta as a function of gestational age. J Clin Endocrinol Metab 81: 2154–2158
Zeold A et al. (2006) Metabolic instability of type 2 deiodinase is transferable to stable proteins independently of subcellular localization. J Biol Chem 281: 31538–31543
Friesema EC et al. (2006) Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol Endocrinol 20: 2761–2772
Baqui M et al. (2003) Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem 278: 1206–1211
Mortimer RH et al. (1996) Maternal to fetal thyroxine transmission in the human term placenta is limited by inner ring deiodination. J Clin Endocrinol Metab 81: 2247–2249
Hernandez A et al. (2006) Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116: 476–484
Stanley EL et al. (2001) Differential expression of sulfotransferase enzymes involved in thyroid hormone metabolism during human placental development. J Clin Endocrinol Metab 86: 5944–5955
Kester MH et al. (2002) Characterization of iodothyronine sulfatase activities in human and rat liver and placenta. Endocrinology 143: 814–819
McKinnon B et al. (2005) Synthesis of thyroid hormone binding proteins transthyretin and albumin by human trophoblast. J Clin Endocrinol Metab 90: 6714–6720
Moestrup SK et al. (1996) Megalin-mediated endocytosis of transcobalamin-vitamin-B12 complexes suggests a role of the receptor in vitamin-B12 homeostasis. Proc Natl Acad Sci USA 93: 8612–8617
Lisi S et al. (2003) Preferential megalin-mediated transcytosis of low-hormonogenic thyroglobulin: a control mechanism for thyroid hormone release. Proc Natl Acad Sci USA 100: 14858–14863
Kilby MD et al. (1998) Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR). J Clin Endocrinol Metab 83: 2964–2971
Banovac K et al. (1986) Triiodothyronine (T3) nuclear binding sites in human placenta and decidua. Placenta 7: 543–549
Emerson CH et al. (1988) The effect of thyroid dysfunction and fasting on placenta inner ring deiodinase activity in the rat. Endocrinology 122: 809–816
Yoshida K et al. (1985) Human placental thyroxine inner ring monodeiodinase in complicated pregnancy. Metabolism 34: 535–538
Santini F et al. (1993) A study of the serum 3,5,3′-triiodothyronine sulfate concentration in normal and hypothyroid fetuses at various gestational stages. J Clin Endocrinol Metab 76: 1583–1587
Hidal JT and Kaplan MM (1985) Characteristics of thyroxine 5′-deiodination in cultured human placental cells: regulation by iodothyronines. J Clin Invest 76: 947–955
Thorpe-Beeston JG et al. (1991) Thyroid function in small for gestational age fetuses. Obstet Gynecol 77: 701–706
Kilby MD et al. (2000) Expression of thyroid receptor isoforms in the human fetal central nervous system and the effects of intrauterine growth restriction. Clin Endocrinol (Oxf) 53: 469–477
Jansson T and Powell TL (2006) IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor?—a review. Placenta 27 (Suppl A): S91–S97
Singh PK et al. (2003) Establishment of reference intervals for markers of fetal thyroid status in amniotic fluid. J Clin Endocrinol Metab 88: 4175–4179
Friesema EC et al. (2008) Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol 22: 1357–1369
Acknowledgements
The authors acknowledge the significant contribution made to their work in the human placenta by their long-standing collaborators, Professor J Franklyn and Dr C McCabe. The authors' research is funded by the Health Foundation, the Medical Research Council (UK), Action Medical Research, and the University of Birmingham. Competing interests
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chan, S., Vasilopoulou, E. & Kilby, M. The role of the placenta in thyroid hormone delivery to the fetus. Nat Rev Endocrinol 5, 45–54 (2009). https://doi.org/10.1038/ncpendmet1026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet1026
This article is cited by
-
The maternal effects of dietary restriction on Dnmt expression and reproduction in two clones of Daphnia pulex
Heredity (2023)
-
Role and Clinical Significance of Monocarboxylate Transporter 8 (MCT8) During Pregnancy
Reproductive Sciences (2023)
-
Relationship between thyroid hormones and central nervous system metabolism in physiological and pathological conditions
Pharmacological Reports (2022)
-
Regional effect on the molecular clock rate of protein evolution in Eutherian and Metatherian genomes
BMC Ecology and Evolution (2021)
-
A pathway level analysis of PFAS exposure and risk of gestational diabetes mellitus
Environmental Health (2021)