Abstract
Glucocorticoids are taken by approximately 2% of the US adult population at any given time. The powerful anti-inflammatory and immunosuppressive benefits of these drugs must, however, be weighed against their multisystem adverse effects. Clinicians should always prescribe the lowest possible dose for the shortest possible time. Patients should be informed of the short-term and long-term adverse effects to expect, particularly if the dose of glucocorticoids is expected to exceed the equivalent of approximately 7.5 mg prednisone daily for 2 months or more. At the commencement of glucocorticoid therapy, a patient's blood pressure, lipid profile, 25-hydroxyvitamin D3 level and fasting glucose level should be measured and baseline bone densitometry performed. Bisphosphonate therapy should be initiated for postmenopausal women and men with a bone density T-score below −1 or for those with a history of fracture. Regular ophthalmic screening for cataracts and glaucoma is warranted, and patients at high-risk of gastric ulceration (especially patients simultaneously taking nonsteroidal anti-inflammatory drugs) should receive proton-pump inhibitors. Prophylaxis against opportunistic infections is appropriate for high-risk populations, such as organ-transplant recipients. Trimethoprim plus sulfamethoxazole can be given to high-risk populations, such as organ transplant recipients. The duration of weaning from glucocorticoid treatment should be proportionate to treatment duration. Appropriate preventive therapy can mitigate many of the adverse effects associated with glucocorticoid therapy.
Key Points
-
Glucocorticoids should be prescribed at the lowest possible dose for the shortest possible time
-
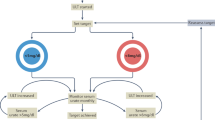
Appropriate baseline tests for patients initiating chronic glucocorticoid therapy include lipid profile, vitamin D3 level, fasting glucose level, and bone densitometry
-
Bisphosphonate therapy is appropriate for glucocorticoid-treated men or postmenopausal women who have a bone density T-score of less than −1
-
Prophylaxis against opportunistic infections is appropriate for high-risk patients
-
The duration of weaning from glucocorticoids should correlate with the duration of treatment
-
Proton-pump inhibitors are appropriate for patients receiving concurrent nonsteroidal anti-inflammatory drugs
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ettinger B et al. (2001) Who bears responsibility for glucocorticoid-exposed patients in a large health maintenance organization. J Manag Care Pharm 7: 228–232
van Staa TP et al. (2000) Use of oral corticosteroids in the United Kingdom. QJM 93: 105–111
Esteban NV et al. (1991) Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 72: 39–45
van Staa TP et al. (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13: 777–787
McDougall R et al. (1994) Outcome in patients with rheumatoid arthritis receiving prednisone compared to matched controls. J Rheumatol 21: 1207–1213
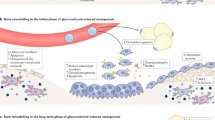
Canalis E (2005) Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep 3: 98–102
Liu Y et al. (2004) Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. J Bone Miner Res 19: 479–490
O'Brien CA et al. (2004) Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145: 1835–1841
Hofbauer LC et al. (1999) Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 140: 4382–4389
Weinstein RS (2007) Is long-term glucocorticoid therapy associated with a high prevalence of asymptomatic vertebral fractures. Nat Clin Pract Endocrinol Metab 3: 86–87
van Staa TP (2006) The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 79: 129–137
Canalis E et al. (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18: 1319–1328
Van Staa TP et al. (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15: 993–1000
Angeli A et al. (2006) High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone 39: 253–259
Van Staa TP et al. (2003) Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48: 3224–3229
No authors listed (2001) Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Arthritis Rheum 44: 1496–1503
Eastell R et al. (1998) A UK Consensus Group on management of glucocorticoid-induced osteoporosis: an update. J Intern Med 244: 271–292
Plotkin LI et al. (1999) Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 104: 1363–1374
Reid DM et al. (2000) Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res 15: 1006–1013
Wallach S et al. (2000) Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 67: 277–285
de Nijs RN et al. (2006) Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med 355: 675–684
Saag KG et al. (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357: 2028–2039
Vreden SG et al. (1991) Aseptic bone necrosis in patients on glucocorticoid replacement therapy. Neth J Med 39: 153–157
Kubo T et al. (2007) Clinical condition of steroid-induced osteonecrosis of the femoral head. Clin Calcium 17: 856–862
Nagasawa K et al. (2005) Very early development of steroid-associated osteonecrosis of femoral head in systemic lupus erythematosus: prospective study by MRI. Lupus 14: 385–390
Dropcho EJ and Soong SJ (1991) Steroid-induced weakness in patients with primary brain tumors. Neurology 41: 1235–1239
Batchelor TT et al. (1997) Steroid myopathy in cancer patients. Neurology 48: 1234–1238
Weiner P et al. (1993) The effect of corticosteroids on inspiratory muscle performance in humans. Chest 104: 1788–1791
Afifi AK et al. (1968) Steroid myopathy. Clinical, histologic and cytologic observations. Johns Hopkins Med J 123: 158–173
Kanda F et al. (2001) Steroid myopathy: pathogenesis and effects of growth hormone and insulin-like growth factor-I administration. Horm Res 56 (Suppl 1): S24–S28
Falduto MT et al. (1992) Exercise inhibits glucocorticoid-induced glutamine synthetase expression in red skeletal muscles. Am J Physiol 262: C214–C220
Falduto MT et al. (1992) Exercise interrupts ongoing glucocorticoid-induced muscle atrophy and glutamine synthetase induction. Am J Physiol 263: E1157–E1163
Kanda F et al. (1999) Preventive effects of insulin-like growth factor-I on steroid-induced muscle atrophy. Muscle Nerve 22: 213–217
Kimura K et al. (2001) Insulin-like growth factor 1 inhibits glucocorticoid-induced glutamine synthetase activity in cultured L6 rat skeletal muscle cells. Neurosci Lett 302: 154–156
Stitt TN et al. (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403
Bowyer SL et al. (1985) Steroid myopathy: incidence and detection in a population with asthma. J Allergy Clin Immunol 76: 234–242
Khaleeli AA et al. (1983) Corticosteroid myopathy: a clinical and pathological study. Clin Endocrinol (Oxf) 18: 155–166
Braunstein PW Jr and DeGirolami U (1981) Experimental corticosteroid myopathy. Acta Neuropathol (Berl) 55: 167–172
Wolfe F et al. (2006) Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum 54: 628–634
Ginzler E et al. (1978) Computer analysis of factors influencing frequency of infection in systemic lupus erythematosus. Arthritis Rheum 21: 37–44
Stuck AE et al. (1989) Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis 11: 954–963
Segal BH and Sneller MC (1997) Infectious complications of immunosuppressive therapy in patients with rheumatic diseases. Rheum Dis Clin North Am 23: 219–237
Ehrchen J et al. (2007) Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood 109: 1265–1274
Gluck T et al. (2005) Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J Rheumatol 32: 1473–1480
Schiff D (1996) Pneumocystis pneumonia in brain tumor patients: risk factors and clinical features. J Neurooncol 27: 235–240
Slivka A et al. (1993) Pneumocystis carinii pneumonia during steroid taper in patients with primary brain tumors. Am J Med 94: 216–219
Yale SH and Limper AH (1996) Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc 71: 5–13
Henson JW et al. (1991) Pneumocystis carinii pneumonia in patients with primary brain tumors. Arch Neurol 48: 406–409
Rodriguez M and Fishman JA (2004) Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev 17: 770–872
Cline JC and Davis SM (1997) Risks of infection or reactivation of tuberculosis associated with chronic corticosteroid therapy. Ann Pharmacother 31: 775–776
Kobashi Y and Matsushima T (2002) Clinical analysis of pulmonary tuberculosis in association with corticosteroid therapy. Intern Med 41: 1103–1110
Fauci AS et al. (1976) Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 84: 304–315
Messer J et al. (1983) Association of adrenocorticosteroid therapy and peptic-ulcer disease. N Engl J Med 309: 21–24
Conn HO and Poynard T (1994) Corticosteroids and peptic ulcer: meta-analysis of adverse events during steroid therapy. J Intern Med 236: 619–632
Pecora PG and Kaplan B (1996) Corticosteroids and ulcers: is there an association. Ann Pharmacother 30: 870–872
Piper JM et al. (1991) Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med 114: 735–740
Garcia EB et al. (2006) Gastrointestinal prophylactic therapy among patients with arthritis treated by rheumatology specialists. J Rheumatol 33: 779–784
Wei L et al. (2004) Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 141: 764–770
Walker BR (2007) Glucocorticoids and cardiovascular disease. Eur J Endocrinol 157: 545–559
Sudhir K et al. (1989) Hydrocortisone-induced hypertension in humans: pressor responsiveness and sympathetic function. Hypertension 13: 416–421
Henkin Y et al. (1992) Secondary dyslipidemia. Inadvertent effects of drugs in clinical practice. JAMA 267: 961–968
Uzu T et al. (2007) Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract 105: c54–c57
van Rossum EF and Lamberts SW (2004) Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res 59: 333–357
Del Rosso J and Friedlander SF (2005) Corticosteroids: options in the era of steroid-sparing therapy. J Am Acad Dermatol 53: S50–S58
Shubin H (1965) Long term (five or more years) administration of corticosteroids in pulmonary diseases. Dis Chest 48: 287–290
Talas DU et al. (2002) The effects of corticosteroids on the healing of tracheal anastomoses in a rat model. Pharmacol Res 45: 299–304
Eubanks TR et al. (1997) The effects of different corticosteroids on the healing colon anastomosis and cecum in a rat model. Am Surg 63: 266–269
Talas DU et al. (2002) The effects of dexamethasone on lipid peroxidation and nitric oxide levels on the healing of tracheal anastomoses: an experimental study in rats. Pharmacol Res 46: 265–271
Yol S et al. (2000) Effect of pedunculated seromuscular flap on bursting strength of intestinal anastomosis after corticosteroid treatment. Dis Colon Rectum 43: 987–990
Raboff W et al. (1994) Mesh reinforcement increases bursting strength of intestinal anastomoses in steroid-treated rabbits. Am Surg 60: 721–727
Karagas MR et al. (2001) Non-melanoma skin cancers and glucocorticoid therapy. Br J Cancer 85: 683–686
Sorensen HT et al. (2004) Skin cancers and non-Hodgkin lymphoma among users of systemic glucocorticoids: a population-based cohort study. J Natl Cancer Inst 96: 709–711
Louis DS et al. (1986) Cutaneous atrophy after corticosteroid injection. Am Fam Physician 33: 183–186
Jacobs MB (1986) Local subcutaneous atrophy after corticosteroid injection. Postgrad Med 80: 159–160
Jones R III and Rhee DJ (2006) Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol 17: 163–167
Garbe E et al. (1997) Risk of ocular hypertension or open-angle glaucoma in elderly patients on oral glucocorticoids. Lancet 350: 979–982
Bouzas EA et al. (2002) Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol 47: 431–448
Brown ES et al. (1999) The psychiatric side effects of corticosteroids. Ann Allergy Asthma Immunol 83: 495–503
Brown ES and Suppes T (1998) Mood symptoms during corticosteroid therapy: a review. Harv Rev Psychiatry 5: 239–246
Denburg SD et al. (1994) Corticosteroids and neuropsychological functioning in patients with systemic lupus erythematosus. Arthritis Rheum 37: 1311–1320
van Rossum EF et al. (2006) Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry 59: 681–688
Hall RC et al. (1979) Presentation of the steroid psychoses. J Nerv Ment Dis 167: 229–236
Pepin MC et al. (1992) Antidepressant drug action in a transgenic mouse model of the endocrine changes seen in depression. Mol Pharmacol 42: 991–995
Pepin MC et al. (1992) Increased glucocorticoid receptor gene promoter activity after antidepressant treatment. Mol Pharmacol 41: 1016–1022
Calfa G et al. (2003) Characterization and functional significance of glucocorticoid receptors in patients with major depression: modulation by antidepressant treatment. Psychoneuroendocrinology 28: 687–701
Pariante CM et al. (1995) Glucocorticoid receptors in depression. Isr J Med Sci 31: 705–712
Brown ES et al. (2003) Effect of lamotrigine on mood and cognition in patients receiving chronic exogenous corticosteroids. Psychosomatics 44: 204–208
Brown ES et al. (2004) An open-label trial of olanzapine for corticosteroid-induced mood symptoms. J Affect Disord 83: 277–281
Kuhlmann S and Wolf OT (2005) Cortisol and memory retrieval in women: influence of menstrual cycle and oral contraceptives. Psychopharmacology (Berl) 183: 65–71
Het S et al. (2005) A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 30: 771–784
Brown ES et al. (2006) Effect of two prednisone exposures on mood and declarative memory. Neurobiol Learn Mem 86: 28–34
Keenan PA et al. (1996) The effect on memory of chronic prednisone treatment in patients with systemic disease. Neurology 47: 1396–1402
Bernal-Mizrachi C et al. (2003) Dexamethasone induction of hypertension and diabetes is PPAR-α dependent in LDL receptor-null mice. Nat Med 9: 1069–1075
Bernal-Mizrachi C et al. (2007) An afferent vagal nerve pathway links hepatic PPARα activation to glucocorticoid-induced insulin resistance and hypertension. Cell Metab 5: 91–102
Gurwitz JH et al. (1994) Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med 154: 97–101
Nabhan FA (2007) Hyperglycemia in the hospital setting. N Engl J Med 356: 753
Braithwaite SS et al. (1998) Managing diabetes during glucocorticoid therapy. How to avoid metabolic emergencies. Postgrad Med 104: 163–166, 171, 175–176
Hoogwerf B and Danese RD (1999) Drug selection and the management of corticosteroid-related diabetes mellitus. Rheum Dis Clin North Am 25: 489–505
LaRochelle GE Jr et al. (1993) Recovery of the hypothalamic-pituitary-adrenal (HPA) axis in patients with rheumatic diseases receiving low-dose prednisone. Am J Med 95: 258–264
Nichols T et al. (1965) Diurnal variation in suppression of adrenal function by glucocorticoids. J Clin Endocrinol Metab 25: 343–349
Axelrod L (1976) Glucocorticoid therapy. Medicine (Baltimore) 55: 39–65
Papanicolaou DA et al. (1996) Acute glucocorticoid deficiency is associated with plasma elevations of interleukin-6: does the latter participate in the symptomatology of the steroid withdrawal syndrome and adrenal insufficiency. J Clin Endocrinol Metab 81: 2303–2306
Streck WF and Lockwood DH (1979) Pituitary adrenal recovery following short-term suppression with corticosteroids. Am J Med 66: 910–914
Graber AL et al. (1965) Natural history of pituitary-adrenal recovery following long-term suppression with corticosteroids. J Clin Endocrinol Metab 25: 11–16
Krasner AS (1999) Glucocorticoid-induced adrenal insufficiency. JAMA 282: 671–676
Byyny RL (1976) Withdrawal from glucocorticoid therapy. N Engl J Med 295: 30–32
May ME and Carey RM (1985) Rapid adrenocorticotropic hormone test in practice. Retrospective review. Am J Med 79: 679–684
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Trikudanathan, S., McMahon, G. Optimum management of glucocorticoid-treated patients. Nat Rev Endocrinol 4, 262–271 (2008). https://doi.org/10.1038/ncpendmet0791
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0791
This article is cited by
-
Opportunistic infection in patients with acute liver failure
Hepatology International (2014)
-
Evaluation of Efficacy, Biodistribution, and Inflammation for a Potent siRNA Nanoparticle: Effect of Dexamethasone Co-treatment
Molecular Therapy (2010)
-
Opportunistic infection in the patients with acute liver failure: a report of three cases with one fatality
Clinical Journal of Gastroenterology (2009)
-
Glukokortikoide in der Rheumatologie
Zeitschrift für Rheumatologie (2008)