Abstract
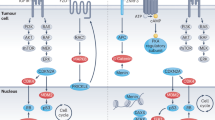
Most adrenocortical tumors are benign, unilateral, adrenocortical adenomas that are often discovered incidentally. Adrenocortical cancer is rare. Exceptionally, adrenocortical tumors can be bilateral. Although most adrenocortical tumors occur sporadically, they may also feature in congenital and/or familial disease. The identification of germline genetic defects in familial diseases associated with adrenocortical tumors helped to define the somatic alterations in sporadic disease: for example, overexpression of insulin-like growth factor 2 and alterations at the 11p15 locus (observed in Beckwith–Wiedemann syndrome) are also found in most adrenocortical cancers. Similarly, inactivating mutations of the TP53 gene, located at 17p13 (observed in Li–Fraumeni syndrome), can also be found at the somatic level in sporadic adrenocortical cancers, as can 17p13 allelic losses. Components of the cyclic AMP signaling pathway—for example, adrenocorticotropic hormone receptors and other membrane receptors, Gs proteins and protein kinase A—can be altered to various degrees in adrenocortical tumors. More recently, gene profiling and genetic studies have shown that the Wnt–β-catenin signaling pathway is frequently activated in adrenocortical tumors. These research findings already have profound implications for clinical management of patients with adrenocortical tumors, for example in unraveling the genetic origin of the disease in some patients, and in the development of molecular markers for diagnosis and prognosis. The new findings should also help in the development of new therapeutic options.
Key Points
-
The 17p13 (for TP53, tumor-suppressor p53) and 11p15 (for IGF2, insulin-like growth factor 2) loci are chromosomal regions that are involved in some rare forms of familial adrenocortical tumors
-
17p13 and 11p15 are frequently altered in sporadic adrenocortical cancers
-
Various components of the cyclic AMP signaling pathway (receptors, Gs proteins, protein kinase A) can be activated in benign, secreting, sporadic and/or familial adrenocortical tumors
-
The Wnt–β-catenin signaling pathway can be activated in benign and malignant adrenocortical tumors
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grumbach MM et al. (2003) Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med 138: 424–429
Luton JP et al. (1990) Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med 322: 1195–1201
Coulter LC (2005) Fetal adrenal develoment: insight gained from adrenal tumors. Trends Endocrinol Metab 16: 235–242
Else T and Hammer GD (2005) Genetics analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab 16: 458–468
Gicquel C et al. (1994) Clonal analysis of human adrenocortical carcinomas and secreting adenomas. Clin Endocrinol (Oxf) 40: 465–477
Beuschlein F et al. (1994) Clonal composition of human adrenocortical neoplasms. Cancer Res 54: 4927–4932
Kjellman M et al. (1999) Genotyping of adrenocortical tumors: very frequent deletions of the MEN1 locus in 11q13 and of a 1-centimorgan region in 2p16. J Clin Endocrinol Metab 84: 730–735
Sidhu S et al. (2002) Comparative genomic hybridization analysis of adrenocortical tumors. J Clin Endocrinol Metab 87: 3467–3474
Bertherat J and Gimenez-Roqueplo AP (2005) New insights in the genetics of adrenocortical tumors, pheochromocytomas and paragangliomas. Horm Metab Res 37: 384–390
Hisada M et al. (1998) Multiple primary cancers in families with Li–Fraumeni syndrome. J Natl Cancer Inst 90: 606–611
Varley JM et al. (1999) Are there low-penetrance TP53 alleles? Evidence from childhood adrenocortical tumors. Am J Hum Genet 65: 995–1006
Ribeiro RC et al. (2001) An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci USA 98: 9330–9335
Latronico AC et al. (2001) An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J Clin Endocrinol Metab 86: 4970–4973
DiGiammarino EL et al. (2002) A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat Struct Biol 9: 12–16
Libe R and Bertherat J (2005) Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol 153: 477–487
Gicquel C et al. (2001) Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res 61: 6762–6767
Gaston V et al. (2001) Analysis of the methylation status of the KCNQ10T and H19 genes in leukocyte DNA for the diagnosis and prognosis of Beckwith–Wiedemann syndrome. Eur J Hum Genet 9: 409–418
Giordano TJ et al. (2003) Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol 162: 521–531
de Fraipont F et al. (2005) Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J Clin Endocrinol Metab 90: 1819–1829
Thakker RV (1998) Multiple endocrine neoplasia—syndromes of the twentieth century. J Clin Endocrinol Metab 83: 2617–2620
Schulte KM et al. (2000) Complete sequencing and messenger ribonucleic acid expression analysis of the MEN I gene in adrenal cancer. J Clin Endocrinol Metab 85: 441–448
Carney JA et al. (1985) The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64: 270–283
Kirschner LS et al. (2000) Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the Carney complex. Hum Mol Genet 9: 3037–3046
Kirschner LS et al. (2000) Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26: 89–92
Groussin L et al. (2002) Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology. Am J Hum Genet 71: 1432–1442
Groussin L et al. (2002) Mutations of the PRKAR1A gene in Cushing's syndrome due to sporadic primary pigmented nodular adrenocortical disease. J Clin Endocrinol Metab 87: 4324–4329
Bertherat J et al. (2003) Molecular and functional analysis of PRKAR1A and its locus (17q22–24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res 63: 5308–5319
Swords FM et al. (2002) Impaired desensitization of a mutant adrenocorticotropin receptor associated with apparent constitutive activity. Mol Endocrinol 16: 2746–2753
Reincke M et al. (1997) Deletion of the adrenocorticotropin receptor gene in human adrenocortical tumors: implications for tumorigenesis. J Clin Endocrinol Metab 82: 3054–3058
Weinstein LS et al. (1991) Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N Engl J Med 325: 1688–1695
Lumbroso S et al. (2004) Activating Gsα mutations: analysis of 113 patients with signs of McCune–Albright syndrome—a European Collaborative Study. J Clin Endocrinol Metab 89: 2107–2113
Yoshimoto K et al. (1993) Rare mutations of the Gsα subunit gene in human endocrine tumors. Mutation detection by polymerase chain reaction-primer-introduced restriction analysis. Cancer 72: 1386–1393
Dall'Asta C et al. (2004) Assessing the presence of abnormal regulation of cortisol secretion by membrane hormone receptors: in vivo and in vitro studies in patients with functioning and non-functioning adrenal adenoma. Horm Metab Res 36: 578–583
Fragoso MC et al. (2003) Cushing's syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J Clin Endocrinol Metab 88: 2147–2151
Lacroix A et al. (1992) Gastric inhibitory polypeptide-dependent cortisol hypersecretion—a new cause of Cushing's syndrome. N Engl J Med 327: 974–980
Reznik Y et al. (1992) Food-dependent Cushing's syndrome mediated by aberrant adrenal sensitivity to gastric inhibitory polypeptide. N Engl J Med 327: 981–986
Mazzuco TL et al. (2006) Ectopic expression of the gastric inhibitory polypeptide receptor gene is a sufficient genetic event to induce benign adrenocortical tumor in a xenotransplantation model. Endocrinology 147: 782–790
Lacroix A et al. (2001) Ectopic and abnormal hormone receptors in adrenal Cushing's syndrome. Endocr Rev 22: 75–110
Horvath A et al. (2006) A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 38: 794–800
Horvath A et al. (2006) Serial analysis of gene expression in adrenocortical hyperplasia caused by a germline PRKAR1A mutation. J Clin Endocrinol Metab 91: 584–596
Nadella KS and Kirschner LS (2005) Disruption of protein kinase A regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res 65: 10307–10315
Griffin KJ et al. (2004) A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumors: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet 41: 923–931
Robinson-White A et al. (2003) Protein kinase-A activity in PRKAR1A-mutant cells, and regulation of mitogen-activated protein kinases ERK1/2. Hum Mol Genet 12: 1475–1484
Kikuchi A (2003) Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci 94: 225–229
Naylor EW and Gardner EJ (1981) Adrenal adenomas in a patient with Gardner's syndrome. Clin Genet 20: 67–73
Blaker H et al. (2004) Analysis of somatic APC mutations in rare extracolonic tumors of patients with familial adenomatous polyposis coli. Genes Chromosomes Cancer 41: 93–98
Bourdeau I et al. (2004) Gene array analysis of macronodular adrenal hyperplasia confirms clinical heterogeneity and identifies several candidate genes as molecular mediators. Oncogene 23: 1575–1585
Tissier F et al. (2005) Mutations of β-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 65: 7622–7627
Feige JJ et al. (1998) Fine tuning of adrenocortical functions by locally produced growth factors. J Endocrinol 158: 7–19
Xing M et al. (2005) BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90: 6373–6379
Rosenberg D et al. (2002) Role of the PKA-regulated transcription factor CREB in development and tumorigenesis of endocrine tissues. Ann NY Acad Sci 968: 65–74
Stratakis CA et al. (1999) Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med 131: 585–591
Bertherat J et al. (2005) In vivo and in vitro screening for illegitimate receptors in adrenocorticotropin-independent macronodular adrenal hyperplasia causing Cushing's syndrome: identification of two cases of gonadotropin/gastric inhibitory polypeptide-dependent hypercortisolism. J Clin Endocrinol Metab 90: 1302–1310
Kirschner LS (2006) Emerging treatment strategies for adrenocortical carcinoma: a new hope. J Clin Endocrinol Metab 91: 14–21
Acknowledgements
This work was supported in part by the Plan Hospitalier de Recherche Clinique to the Adrenocortical and Medulla Endocrine Tumor (COMETE) network (AOM 02068), the Ligue National Contre le Cancer (Grant 04-7571), the Groupement d'Intérêt Scientifique–Institut National de la Santé et de la Recherche Médicale (GIS–INSERM) Institut des Maladies Rares for the Carney Complex network, and the European Network for the Study of Adrenal Tumors (ENSAT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bertherat, J., Groussin, L. & Bertagna, X. Mechanisms of Disease: adrenocortical tumors—molecular advances and clinical perspectives. Nat Rev Endocrinol 2, 632–641 (2006). https://doi.org/10.1038/ncpendmet0321
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0321