Abstract
It is well known that, in large populations, HDL-cholesterol levels are inversely related to the risk of atherosclerotic clinical events; however, in an individual, the predictive value of an HDL-cholesterol level is far from perfect. As a result, other HDL-associated factors have been investigated, including the quality and function of HDL in contradistinction to the level of HDL-cholesterol. Regarding their quality, HDL particles are highly heterogeneous and contain varying levels of antioxidants or pro-oxidants, which results in variation in HDL function. It has been postulated that HDL functions to promote reverse cholesterol transport. Recent studies support this role for HDL but also indicate that HDL is a modulator of systemic inflammation. In the absence of inflammation, HDL has a complement of antioxidant enzymes that work to maintain an anti-inflammatory state. In the presence of systemic inflammation, these antioxidant enzymes can be inactivated and HDL can accumulate oxidized lipids and proteins that make it proinflammatory. Under these conditions the main protein of HDL, apolipoprotein A-I, can be modified by reactive oxygen species. This modification impairs the ability of HDL to promote cholesterol efflux by the ATP-binding cassette transporter A-1 pathway. Animal studies and small-scale human studies suggest that measures of the quality and novel functions of HDL might provide an improved means of identifying subjects at increased risk for atherosclerotic events, compared with the current practice of only measuring HDL-cholesterol levels. The quality and function of HDL are also attractive targets for emerging therapies.
Key Points
-
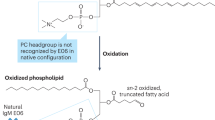
Oxidized lipids mediate inflammatory responses in the artery wall; HDL-associated enzymes can destroy these proinflammatory oxidized lipids, but are also inhibited by them
-
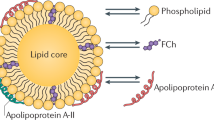
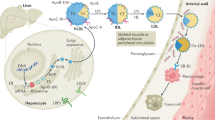
Apolipoprotein A-I (apoA-I) is the main protein in HDL and is critical for promoting reverse cholesterol transport (i.e. from peripheral tissues, such as arteries, back to the liver where the excess cholesterol is excreted in the bile)
-
Oxidants produced by macrophage enzymes, such as myeloperoxidase, can damage apoA-I and reduce its ability to promote reverse cholesterol transport
-
HDL-cholesterol levels predict risk for atherosclerosis in large populations, but many individuals with clinical events due to atherosclerosis have normal or even high HDL-cholesterol levels
-
HDL is anti-inflammatory in the absence of inflammation but can become proinflammatory in the presence of a systemic inflammation (such as atherosclerosis) because of the damage to HDL (specifically, apoA-I and HDL-associated enzymes) caused by the oxidants produced in the inflammatory reaction
-
Novel therapies are being tested that will raise HDL-cholesterol levels and/or improve the anti-inflammatory properties of HDL
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Navab M et al. (2005) The double jeopardy of HDL. Ann Med 37: 173–178
Gordon T et al. (1977) High density lipoprotein as a protective factor against coronary heart disease. Am J Med 62: 707–714
Ansell BJ et al. (2003) Inflammatory/anti-inflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108: 2751–2756
Navab M et al. (2004) The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res 45: 993–1007
Downs JR et al. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279: 1615–1622
Castelli WP et al. (1986) Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham study. JAMA 256: 2835–2838
Ridker PM (2002) On evolutionary biology, inflammation, infection, and the causes of atherosclerosis. Circulation 105: 2–4
Hessler JR et al. (1979) LDL induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis 32: 213–229
Colles SM et al. (2001) Oxidized LDL-induced injury and apoptosis in atherosclerosis. Potential roles for oxysterols. Trends Cardiovasc Med 11: 131–138
Berliner JA and Watson AD (2005) A role for oxidized phospholipids in atherosclerosis. N Engl J Med 353: 9–11
Libby P and Theroux P (2005) Pathophysiology of coronary artery disease. Circulation 111: 3481–3488
Tsimikas S et al. (2005) Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med 353: 46–57
Barter PJ et al. (2004) Anti-inflammatory properties of HDL. Circulation Res 95: 764–772
Zhang Y et al. (2003) Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation 108: 661–663
Moore RE et al. (2005) Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circulation Res 97: 763–771
Navab M et al. (2001) HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscl Thromb Vasc Biol 21: 481–488
Bielicki JK and Forte TM (1999) Evidence that lipid hydroperoxides inhibit plasma lecithin:cholesterol acyltransferase activity. J Lipid Res 40: 948–954
Forte TM et al. (2002) Altered activities of anti-atherogenic enzymes, LCAT, paraoxonase, and platelet-activating factor acetylhydrolase in atherosclerosis-susceptible mice. J Lipid Res 43: 477–485
Van Lenten BJ et al. (1995) Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 96: 2758–2767
Van Lenten BJ et al. (2001) High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation 103: 2283–2288
Nakamura Y et al. (2004) Molecular mechanism of reverse cholesterol transport: reaction of pre-β-migrating high-density lipoprotein with plasma lecithin/cholesterol acyltransferase. Biochemistry 43: 14811–14820
Cavallero E et al. (1995) Abnormal reverse cholesterol transport in controlled type II diabetic patients. Arterioscl Thromb Vasc Biol 15: 2130–2135
Gowri MS et al. (1999) Decreased protection by HDL from poorly controlled type 2 diabetic subjects against LDL oxidation may be due to the abnormal composition of HDL. Arterioscl Thromb Vasc Biol 19: 2226–2233
Zheng L et al. (2004) Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest 114: 529–541
Zheng L et al. (2005) Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J Biol Chem 280: 38–47
Peng DQ et al. (2005) Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J Biol Chem 280: 33775–33784
Pennathur S et al. (2004) Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem 279: 42977–42983
Bergt C et al. (2004) The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA 101: 13032–13037
Navab M et al. (2001) A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res 42: 1308–1317
Navab M et al. (2004) The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res 45: 993–1007
Kisilevsky R and Subramanian L (1992) Serum amyloid A changes high density lipoprotein's cellular affinity. A clue to serum amyloid A's principal function. Lab Invest 67: 151–158
Fogelman AM (2004) When good cholesterol goes bad. Nat Med 10: 902–903
Navab M et al. (2001) High-density lipoprotein and the dynamics of atherosclerotic lesions. Circulation 104: 2386–2387
Shah PK et al. (2001) High-dose recombinant apolipoprotein A-I (Milano) mobilizes tissue cholesterol rapidly, reduces plaque lipid and macrophage content in apolipoprotein E-deficient mice: potential implications for plaque stabilization. Circulation 103: 3047–3050
Duffy D and Rader DJ (2005) Drugs in development: targeting high-density lipoprotein metabolism and reverse cholesterol transport. Curr Opin Cardiol 20: 301–306
Navab M et al. (2002) Oral administration of an apoA-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105: 290–292
Navab M et al. (2004) Oral D-4F causes formation of pre-β high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 109: 3215–3220
Navab M et al. (2005) D-4F and statins synergize to render HDL anti-inflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscl Thromb Vasc Biol 25: 1426–1432
Navab M et al. (2005) Apolipoprotein A-I mimetic peptides. Arterioscl Thromb Vasc Biol 25: 1325–1331
Ou J et al. (2005) Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ Res 97: 1190–1197
Navab M et al. (2005) An apolipoprotein A-I mimetic works best in the presence of apolipoprotein A-I. Circ Res 97: 1085–1086
Navab M et al. (2005) An oral apoJ peptide renders HDL anti-inflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipoprotein E-null mice. Arterioscl Thromb Vasc Biol 25: 1932–1937
Navab M et al. (2005) Oral small peptides render HDL anti-inflammatory in mice and monkeys and reduce atherosclerosis in apoE null mice. Circ Res 97: 524–532
Shah PK and Chyu K-Y (2005) Apolipoprotein A-I mimetic peptides: potential role in atherosclerosis management. Trends Cardiovasc Med 15: 291–296
Acknowledgements
This work was supported by NIH grants HL-30568 to AM Fogelman, HL-34343 to GM Anantharamaiah, and the Laubisch, Castera, and MK Grey Funds at UCLA to AM Fogelman.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M Navab, GM Anantharamaiah, ST Reddy, and AM Fogelman are principals in Bruin Pharma, BJ Ansell is a stockholder in Bruin Pharma, and AM Fogelman is an officer in Bruin Pharma. BJ Van Lenten declared he has no competing interests.
Rights and permissions
About this article
Cite this article
Navab, M., Anantharamaiah, G., Reddy, S. et al. Mechanisms of Disease: proatherogenic HDL—an evolving field. Nat Rev Endocrinol 2, 504–511 (2006). https://doi.org/10.1038/ncpendmet0245
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0245
This article is cited by
-
Both LDL and HDL particle concentrations associate positively with an increased risk of developing microvascular complications in patients with type 2 diabetes: lost protection by HDL (Zodiac-63)
Cardiovascular Diabetology (2023)
-
Effects of disease activity on lipoprotein levels in patients with early arthritis: can oxidized LDL cholesterol explain the lipid paradox theory?
Arthritis Research & Therapy (2020)
-
Long- and short-term association of low-grade systemic inflammation with cardiovascular mortality in the LURIC study
Clinical Research in Cardiology (2020)
-
Functional implications of single nucleotide polymorphisms rs662 and rs854860 on the antioxidative activity of paraoxonase1 (PON1) in patients with rheumatoid arthritis
Clinical Rheumatology (2019)
-
Circulating MiR-374a-5p is a potential modulator of the inflammatory process in obesity
Scientific Reports (2018)