Abstract
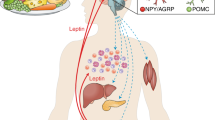
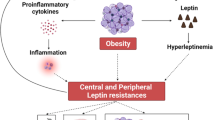
Leptin is an adipocyte-secreted hormone with a key role in energy homeostasis. Studies in animal models, in humans with congenital complete leptin deficiency, and observational and interventional studies in humans with relative leptin deficiency (lower than normal leptin levels) have all indicated that leptin regulates multiple physiological functions, primarily in states of energy deficiency. This information led to proof-of-concept clinical trials involving leptin administration to individuals with relative or complete leptin deficiency. These conditions include congenital complete leptin deficiency, due to mutations in the leptin gene, and states of relative leptin deficiency including lipoatrophy and some forms of hypothalamic amenorrhea. Leptin, in replacement doses, normalizes neuroendocrine, metabolic and immune function in patients with these conditions, but further clinical studies are required to determine its long-term efficacy and safety. Management of leptin-deficient states with replacement doses of leptin holds promise as a therapeutic option. In addition, elucidation of the mechanisms underlying leptin resistance, which characterizes hyperleptinemic states such as human obesity and diabetes, might provide novel therapeutic targets for these prevalent clinical problems.
Key Points
-
The main action of leptin is to regulate multiple physiological functions in states of energy deficiency
-
Common forms of human obesity are characterized by leptin resistance; the mechanisms of this resistance are unknown
-
Leptin therapy is useful in complete congenital leptin deficiency and might be useful in the management of states of relative leptin deficiency, such as hypothalamic amenorrhea and lipodystrophy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Zhang Y et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432
Friedman JM and Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395: 763–770
Heymsfield SB et al. (1999) Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575
Mantzoros CS (1999) The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med 130: 671–680
Matarese G et al. (2005) Leptin in immunology. J Immunol 174: 3137–3142
Zhang F et al. (1997) Crystal structure of the obese protein leptin-E100. Nature 387: 206–209
Licinio J et al. (1997) Human leptin levels are pulsatile and inversely related to pituitary–adrenal function. Nat Med 3: 575–579
Considine RV et al. (1996) Serum immunoreactive leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295
Chan JL et al. (2003) The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111: 1409–1421
Kolaczynski JW et al. (1996) Acute and chronic effects of insulin on leptin production in humans: Studies in vivo and in vitro. Diabetes 45: 699–701
Saad MF et al. (1997) Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab 82: 579–584
Rosenbaum M et al. (1996) Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81: 3424–3427
Lee GH et al. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635
Elmquist JK et al. (1998) Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547
Fei H et al. (1997) Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA 94: 7001–7005
Bjorbaek C et al. (1998) Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology 139: 3485–3491
Schwartz MW et al. (1996) Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2: 589–593
Chan JL et al. (2002) Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes 51: 2105–2112
Ren D et al. (2005) Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab 2: 95–104
Sahu A (2003) Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol 24: 225–253
Schwartz MW and Porte D Jr (2005) Diabetes, obesity, and the brain. Science 307: 375–379
Cowley MA et al. (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484
Pinto S et al. (2004) Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304: 110–115
Feng N et al. (2005) Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes 54: 2663–2667
El-Haschimi K et al. (2003) Leptin resistance—or why leptin fails to work in obesity. Exp Clin Endocrinol Diabetes 111: 2–7
Hallschmid M et al. (2006) Overweight humans are resistant to the weight-reducing effects of melanocortin4–10 . J Clin Endocrinol Metab 91: 522–525
Harris RB et al. (1998) A leptin dose–response study in obese (ob/ob) and lean (+/−) mice. Endocrinology 139: 8–19
Schwartz MW et al. (1996) Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45: 531–535
al Barazanji KA et al. (1997) Effects of intracerebroventricular infusion of leptin in obese Zucker rats. Obes Res 5: 387–394
Garg A and Misra A (2004) Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am 33: 305–331
Shimomura I et al. (1998) Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev 12: 3182–3194
Moitra J et al. (1998) Life without white fat: a transgenic mouse. Genes Dev 12: 3168–3181
Kim JK et al. (2000) Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem 275: 8456–8460
Gavrilova O et al. (2000) Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 105: 271–278
Ebihara K et al. (2001) Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes 50: 1440–1448
Hollander P et al. (2000) A randomized double-blinded placebo-controlled study to determine the effects of subcutaneous recombinant methionyl human leptin on glycemic control in obese subjects with diet-treated type 2 diabetes mellitus. Abstract from the 60th Scientific Sessions of the American Diabetes Association: 2000 June 9–13; San Antonio, TX, USA
Steppan CM et al. (2000) Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92: 73–78
Gordeladze JO et al. (2002) Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 85: 825–836
Cornish J et al. (2002) Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175: 405–415
Ducy P et al. (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100: 197–207
Takeda S et al. (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317
Rahmouni K et al. (2005) Obesity-associated hypertension: new insights into mechanisms. Hypertension 45: 9–14
Welt CK et al. (2004) Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351: 987–997
Farooqi IS et al. (2002) Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110: 1093–1103
Moran SA et al. (2004) Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism 53: 513–519
Chehab FF et al. (1996) Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12: 318–320
Ahima RS et al. (1996) Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252
Licinio J et al. (2004) Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101: 4531–4536
Farooqi IS et al. (1999) Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884
Mantzoros CS et al. (1997) A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab 82: 1066–1070
Oral EA et al. (2002) Effect of leptin replacement on pituitary hormone regulation in patients with severe lipodystrophy. J Clin Endocrinol Metab 87: 3110–3117
Clement K et al. (1998) A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392: 398–401
Ohtake M et al. (1977) Studies on hypothermia and thyroid function in the obese (ob/ob) mouse. Am J Physiol 233: R110–R115
LaPaglia N et al. (1998) Leptin alters the response of the growth hormone releasing factor–growth hormone–insulin-like growth factor-I axis to fasting. J Endocrinol 159: 79–83
Dubuc PU (1977) Basal corticosterone levels of young og/ob mice. Horm Metab Res 9: 95–97
Heiman ML et al. (1997) Leptin inhibition of the hypothalamic–pituitary–adrenal axis in response to stress. Endocrinology 138: 3859–3863
Heiman ML et al. (1997) Leptin inhibition of the hypothalamic–pituitary–adrenal axis in response to stress. Endocrinology 138: 3859–3863
Mancuso P et al. (2002) Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol 168: 4018–4024
Loffreda S et al. (1998) Leptin regulates proinflammatory immune responses. FASEB J 12: 57–65
Caldefie-Chezet F et al. (2003) Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res 37: 809–814
Lord GM et al. (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394: 897–901
Howard JK et al. (1999) Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 104: 1051–1059
Chan JL et al. (2005) Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-α receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab 90: 1625–1631
Canavan B et al. (2005) Effects of physiological leptin administration on markers of inflammation, platelet activation, and platelet aggregation during caloric deprivation. J Clin Endocrinol Metab 90: 5779–5785
Mantzoros CS et al. (1997) Leptin concentrations in relation to body mass index and the tumor necrosis factor-α system in humans. J Clin Endocrinol Metab 82: 3408–3413
Chan JL et al. (2005) Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab 90: 1618–1624
Farooqi IS and O'Rahilly S (2005) Monogenic obesity in humans. Annu Rev Med 56: 443–458
Petersen KF et al. (2002) Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109: 1345–1350
Fogteloo AJ et al. (2003) Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr Metab 16: 109–114
Lejeune MP et al. (2003) Effect of dietary restraint during and following pegylated recombinant leptin (PEG-OB) treatment of overweight men. Int J Obes Relat Metab Disord 27: 1494–1499
Rosenbaum M et al. (2005) Low dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586
Miller KK et al. (1998) Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab 83: 2309–2312
Oral EA et al. (2002) Leptin-replacement therapy for lipodystrophy. N Engl J Med 346: 570–578
Javor ED et al. (2005) Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes 54: 1994–2002
Ebihara K et al. (2004) Long-term leptin-replacement therapy for lipoatrophic diabetes. N Engl J Med 351: 615–616
Musso C et al. (2005) The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism 54: 255–263
Grinspoon S and Carr A (2005) Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 352: 48–62
Lee JH et al. (2006) r-metHuLeptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy (HAART). J Clin Endocrinol Metab in press
Hegyi K et al. (2004) Leptin-induced signal transduction pathways. Cell Biol Int 28: 159–169
Niswender KD et al. (2004) Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab 15: 362–369
Maroni P et al. (2005) Intracellular signal transduction pathways induced by leptin in C2C12 cells. Cell Biol Int 29: 542–550
Chan JL and Mantzoros CS (2005) Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 366: 74–85
Acknowledgements
This work was supported by NIH Grant DK-58785. CS Mantzoros is supported by a Bessel Award from the Alexander von Humboldt Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Christos S Mantzoros has received grant support for clinical research projects from Amgen Inc.
Rights and permissions
About this article
Cite this article
Brennan, A., Mantzoros, C. Drug Insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat Rev Endocrinol 2, 318–327 (2006). https://doi.org/10.1038/ncpendmet0196
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0196
This article is cited by
-
Leptin promoter methylation in female patients with painful multisomatoform disorder and chronic widespread pain
Clinical Epigenetics (2022)
-
A comprehensive review of the impact of obesity on plasma cell disorders
Leukemia (2022)
-
Failure of subcutaneous lipectomy to combat metabolic dysregulations in ovariectomy-induced obesity in young female rats
Hormones (2022)
-
A systematic review of resting-state functional connectivity in obesity: Refining current neurobiological frameworks and methodological considerations moving forward
Reviews in Endocrine and Metabolic Disorders (2022)
-
Mouse models of nonalcoholic steatohepatitis and their application to new drug development
Archives of Pharmacal Research (2022)