Abstract
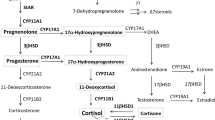
The action of the peptide hormone adrenocorticotropin (ACTH) to stimulate glucocorticoid production by the adrenal gland is an essential physiologic process, yet is dependent on a single unique genetic component—the ACTH receptor or melanocortin 2 receptor. Genetic defects that cause abnormalities in this receptor or in a protein required for its expression at the cell surface result in a potentially fatal disease (familial glucocorticoid deficiency). Overexpression of this receptor or inability to desensitize it is found in adrenal adenomas or hyperplasia associated with glucocorticoid overproduction (Cushing syndrome). These disorders are uncommon, but there are considerable data to show that the hypothalamo–pituitary–adrenal axis is overactive, or in some circumstances underactive, in more common situations including depressive illness and septic shock. The origin of these latter disturbances is undoubtedly complex and multifactorial, but there is good evidence that a component of this phenomenon is an altered responsiveness of the ACTH receptor to ACTH. Understanding the basis of ACTH responsiveness might, therefore, contribute to the understanding of disorders such as these and perhaps enable the hypothalamo–pituitary–adrenal axis to be manipulated beneficially in these circumstances.
Key Points
-
Adrenocorticotropin (ACTH) has a key role in mediating the stress response by acting on a unique membrane receptor in the adrenal gland to stimulate glucocorticoid production
-
Inactivating mutations in the ACTH receptor, or in an accessory protein essential for its expression on the cell surface, cause an inherited syndrome of ACTH insensitivity—familial glucocorticoid deficiency
-
Disturbances of ACTH-receptor expression or function are often found in benign and malignant adrenal neoplasms
-
Altered responsiveness to ACTH is often present as a secondary phenomenon in several common disorders including depressive illness and septic shock
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Berthet J et al. (1957) The relationship of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J Biol Chem 224: 463–475
Haynes RC Jr (1958) The activation of adrenal phosphorylase by the adrenocorticotropic hormone. J Biol Chem 233: 1220–1222
Haynes RC Jr et al. (1959) Influence of adenosine 3″,5″-monophosphate on corticoid production by rat adrenal glands. J Biol Chem 234: 1421–1423
Schimmer BP (1995) Molecular and genetic approaches to the study of signal transduction in the adrenal cortex. Can J Physiol Pharmacol 73: 1097–1107
Lefkowitz RJ et al. (1970) ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci USA 65: 745–752
Lefkowitz RJ et al. (1970) Effects of calcium on ACTH stimulation of the adrenal: separation of hormone binding from adenyl cyclase activation. Nature 228: 864–866
Hofmann K et al. (1988) Identification of a protein in adrenal particulates that binds adrenocorticotropin specifically and with high affinity. Endocrinology 123: 1355–1363
Ramachandran J and Suyama AT (1975) Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci USA 72: 113–117
Mountjoy KG et al. (1992) The cloning of a family of genes that encode melanocortin receptors. Science 257: 1248–1251
Cone RD et al. (1993) Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann N Y Acad Sci 680: 342–363
Cone RD et al. (1996) The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res 51: 287–317
Schioth HB (2001) The physiological role of melanocortin receptors. Vitam Horm 63: 195–232
Enyeart JJ (2005) Biochemical and ionic signaling mechanisms for ACTH-stimulated cortisol production. Vitam Horm 70: 265–279
Bicknell AB et al. (2001) Characterization of a serine protease that cleaves pro-γ-melanotropin at the adrenal to stimulate growth. Cell 105: 903–912
Coll AP et al. (2004) The effects of proopiomelanocortin deficiency on murine adrenal development and responsiveness to adrenocorticotropin. Endocrinology 145: 4721–4727
Arola J et al. (1993) Biphasic effect of ACTH on growth of rat adrenocortical cells in primary culture. Cell Tissue Res 271: 169–176
Le T and Schimmer BP (2001) The regulation of MAPKs in Y1 mouse adrenocortical tumor cells. Endocrinology 142: 4282–4287
Shepard TH et al. (1959) Familial Addison's disease. Am J Dis Child 97: 154–162
Elias LLK et al. (2000) Tall stature in familial glucocorticoid deficiency. Clin Endocrinol 53: 423–430
Imamine H et al. (2005) Possible relationship between elevated plasma ACTH and tall stature in familial glucocorticoid deficiency. Tohoku J Exp Med 205: 123–131
Dumont LM et al. (2005) Evidence for direct actions of melanocortin peptides on bone metabolism. Peptides 26: 1929–1935
Zhong Q et al. (2005) Multiple melanocortin receptors are expressed in bone cells. Bone 36: 820–831
Clark AJL et al. (2005) Inherited ACTH insensitivity illuminates the mechanisms of ACTH action. Trends Endocrinol Metab 16: 451–457
Naville D et al. (1996) Demonstration by transfection studies that mutations in the adrenocorticotropin receptor gene are one cause of the hereditary syndrome of glucocorticoid deficiency. J Clin Endocrinol Metab 81: 1442–1448
Rached M et al. (2005) Expression of the human melanocortin-2 receptor in different eukaryotic cells. Peptides 26: 1842–1847
Elias LLK et al. (1999) Functional characterization of naturally occurring mutations of the human adrenocorticotropin receptor: poor correlation of phenotype and genotype. J Clin Endocrinol Metab 84: 2766–2770
Noon LA et al. (2002) Failed export of the adrenocorticotropin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J Endocrinol 174: 17–25
McLatchie LM et al. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393: 333–339
Saito H et al. (2004) RTP family members induce functional expression of mammalian odorant receptors. Cell 119: 679–691
Metherell LA et al. (2005) Mutations in MRAP, encoding a novel interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency Type 2. Nat Genet 37: 166–170
Génin E et al. (2002) Linkage of one gene for familial glucocorticoid deficiency type 2 (FGD2) to chromosome 8q and further evidence of heterogeneity. Hum Genet 111: 428–434
Light K et al. (1995) Are activating mutations of the ACTH receptor involved in adrenal cortical neoplasia? Life Sci 56: 1523–1527
Latronico AC et al. (1995) No evidence for oncogenic mutations in the adrenocorticotropin receptor gene in human adrenocortical neoplasms. J Clin Endocrinol Metab 80: 875–877
Swords FM et al. (2003) Impaired desensitization of a mutant adrenocorticotropin receptor associated with apparent constitutive activity. Mol Endocrinol 16: 2746–2753
Reincke M et al. (1997) Expression of adrenocorticotrophic hormone receptor mRNA in human adrenocortical neoplasms: correlation with P450scc expression. Clin Endocrinol (Oxf) 46: 619–626
Imai T et al. (2001) Expression of adrenocorticotropin receptor gene in adrenocortical adenomas from patients with Cushing syndrome: possible contribution for the autonomous production of cortisol. Ann Surg 234: 85–91
Reincke M et al. (1997) Deletion of the adrenocorticotropin receptor gene in human adrenocortical tumors: implications for tumorigenesis. J Clin Endocrinol Metab 82: 3054–3058
Zwermann O et al. (2004) The role of the ACTH receptor in adrenal tumors: identification of a novel microsatellite marker. Horm Metab Res 36: 406–410
Tsigos C and Chrousos GP (2002) Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53: 865–871
Di Blasio AM et al. (2003) The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf) 59: 68–74
Gold PW et al. (1986) Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. Pathophysiologic and diagnostic implications. N Engl J Med 314: 1329–1335
Brown ES et al. (2004) Association of depression with medical illness: does cortisol play a role? Biol Psychiatry 55: 1–9
Rubin RT et al. (1996) Adrenal gland volume in major depression: relationship to basal and stimulated pituitary–adrenal cortical axis function. Biol Psychiatry 40: 89–97
Amsterdam JD et al. (1989) Enhanced adrenocortical sensitivity to submaximal doses of cosyntropin (α1-24-corticotropin) in depressed patients. Arch Gen Psychiatry 46: 550–554
Penhoat A et al. (1989) Corticotropin positively regulates its own receptors and cAMP response in cultured bovine adrenal cells. Proc Natl Acad Sci USA 86: 4978–4981
Lebrethon MC et al. (1994) Regulation of corticotropin receptor number and messenger RNA in cultured human adrenocortical cells by corticotropin and angiotensin II. J Clin Invest 93: 1828–1833
Parker KJ et al. (2003) Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav 43: 60–66
Annane D et al. (2000) A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 283: 1038–1045
Annane D et al. (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288: 862–871
de Jong FH et al. (1984) Etomidate suppresses adrenocortical function by inhibition of 11β-hydroxylation. J Clin Endocrinol Metab 59: 1143–1147
Keri G et al. (1981) Effects of septic shock plasma on adrenocortical cell function. Life Sci 28: 1917–1923
Catalano RD et al. (1984) Mechanisms of adrenocortical depression during Escherichia coli shock. Arch Surg 119: 145–150
den Brinker M et al. (2005) Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab 90: 5110–5117
Slawik M et al. (2004) Characterization of an adrenocorticotropin (ACTH) receptor promoter polymorphism leading to decreased adrenal responsiveness to ACTH. J Clin Endocrinol Metab 89: 3131–3137
Reisch N et al. (2005) Genetic influence of an ACTH receptor promoter polymorphism on adrenal androgen secretion. Eur J Endocrinol 153: 711–715
Acknowledgements
The Wellcome Trust and the Biotechnology and Biological Sciences Research Council (BBSRC) fund research in our laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Clark, A., Metherell, L. Mechanisms of Disease: the adrenocorticotropin receptor and disease. Nat Rev Endocrinol 2, 282–290 (2006). https://doi.org/10.1038/ncpendmet0165
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0165
This article is cited by
-
SCARB1 downregulation in adrenal insufficiency with Allgrove syndrome
Orphanet Journal of Rare Diseases (2023)
-
Atypical pituitary hormone-target tissue axis
Frontiers of Medicine (2023)
-
LD block disorder-specific pleiotropic roles of novel CRHR1 in type 2 diabetes and depression disorder comorbidity
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
ACTH and Cortisol responses to ghrelin and desmopressin in patients with Cushing’s disease and adrenal enlargement
Journal of Endocrinological Investigation (2010)
-
Genetics of adrenal tumors associated with Cushing's syndrome: a new classification for bilateral adrenocortical hyperplasias
Nature Clinical Practice Endocrinology & Metabolism (2007)