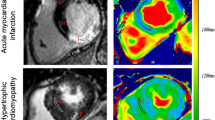
Abstract
Magnetic resonance spectroscopy (MRS) utilizes magnetic resonance signals from nuclei, such as phosphorus-31, to provide information regarding the biochemical composition and metabolic state of cardiac muscle. This technique is the only method available for noninvasive assessment of cardiac metabolism without the need for the application of external radioactive tracers. MRS provides insights into the role of cardiac energetics in ischemic heart disease, heart failure, hypertrophy, and valve disease. Furthermore, response to therapeutic intervention can be monitored using this method. At present, this technique is used as a research tool, because low spatial and temporal resolution, as well as low reproducibility, precludes its diagnostic use in clinical practice; however, higher-field magnetic resonance systems—using, for example, 7 T—will enable improvements in resolution and reproducibility that may take cardiac MRS into the clinical realm.
Key Points
-
Magnetic resonance spectroscopy with phosphorus-31 (31P-MRS) utilizes the nuclear spin of 31P and is the only available method for the noninvasive assessment of cardiac metabolism without need for the application of external radioactive tracers
-
Energetic derangement of the heart in dilated cardiomyopathy predicts prognosis and correlates with left ventricular ejection fraction and NYHA class; improvements in cardiac energetics following pharmacological therapy can be seen using 31P-MRS
-
31P-MRS can differentiate pathological and physiological hypertrophy
-
31P-MRS stress testing can identify exercise-induced ischemia
-
Increased field strengths, such as 3 T or greater, will increase the signal to noise ratio, and will improve the clinical application of 31P-MRS
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Garlick PB et al. (1977) Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun 74: 1256–1262
Ingwall JS (1982) Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol 242: H729–H744
Neubauer S (2003) Cardiac magnetic resonance spectroscopy. In Cardiovascular Magnetic Resonance: Established and Emerging Applications 39–60 (Eds Lardo AC. et al.). London: Taylor and Francis Group
Bottomley PA et al. (1996) Human cardiac high-energy phosphate metabolite concentrations by 1D-resolved NMR spectroscopy. Magn Reson Med 35: 664–670
Bottomley PA et al. (1990) Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn Reson Med 14: 425–434
Bottomley PA (1994) MR spectroscopy of the human heart: the status and the challenges. Radiology 191: 593–612
Pohmann R and von Kienlin M (2001) Accurate phosphorus metabolite images of the human heart by 3D acquisition-weighted CSI. Magn Reson Med 45: 817–826
Lamb HJ et al. (1996) Reproducibility of human cardiac 31P-NMR spectroscopy. NMR Biomed 9: 217–227
Bessman SP and Geiger PJ (1981) Transport of energy in muscle: the phosphorylcreatine shuttle. Science 211: 448–452
Bottomley PA and Weiss RG (2001) Noninvasive localized MR quantification of creatine kinase metabolites in normal and infarcted canine myocardium. Radiology 219: 411–418
Kohler SJ et al. (1991) Analysis of 23Na NMR spectra from isolated perfused hearts. Magn Reson Med 18: 15–27
Horn M et al. (2001) Detection of myocardial viability based on measurement of sodium content: A (23)Na-NMR study. Magn Reson Med 45: 756–764
Sandstede JJ et al. (2001) Assessment of myocardial infarction in humans with (23)Na MR imaging: comparison with cine MR imaging and delayed contrast enhancement. Radiology 221: 222–228
Okada M et al. (1998) Influence of aging or left ventricular hypertrophy on the human heart: contents of phosphorus metabolites measured by 31P MRS. Magn Reson Med 39: 772–782
Conway MA et al. (1988) Cardiac metabolism during exercise measured by magnetic resonance spectroscopy. Lancet 2: 692
Pluim BM et al. (1998) Functional and metabolic evaluation of the athlete's heart by magnetic resonance imaging and dobutamine stress magnetic resonance spectroscopy. Circulation 97: 666–672
Neubauer S (2007) The failing heart an engine out of fuel. N Engl J Med 356: 1140–1151
Neubauer S et al. (1995) Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur Heart J 16 (Suppl): S115–;S118
Neubauer S et al. (1992) 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86: 1810–1818
Neubauer S et al. (1997) Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 96: 2190–2196
Ashrafian H et al. (2003) Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet 19: 263–268
Maron BJ (2002) Hypertrophic cardiomyopathy: a systematic review. JAMA 287: 1308–1320
Crilley JG et al. (2003) Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol 41: 1776–1782
Jung WI et al. (1998) 31P NMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. Circulation 97: 2536–2542
Crilley JG et al. (2000) Magnetic resonance spectroscopy evidence of abnormal cardiac energetics in Xp21 muscular dystrophy. J Am Coll Cardiol 36: 1953–1958
Schocke MF et al. (2004) Cardiac phosphorus-31 two-dimensional chemical shift imaging in patients with hereditary hemochromatosis. Magn Reson Imaging 22: 515–521
Schocke MF et al. (2003) 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors improve myocardial high-energy phosphate metabolism in men. J Cardiovasc Magn Reson 5: 595–602
Lamb HJ et al. (1999) Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation 99: 2261–2267
Clarke K et al. (1987) Temporal relation between energy metabolism and myocardial function during ischemia and reperfusion. Am J Physiol 253: H412–H421
Weiss RG et al. (1990) Regional myocardial metabolism of high-energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med 323: 1593–1600
Yabe T et al. (1995) Quantitative measurements of cardiac phosphorus metabolites in coronary artery disease by 31P magnetic resonance spectroscopy. Circulation 92: 15–23
Buchthal SD et al. (2000) Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 342: 829–835
Johnson BD et al. (2004) Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health–National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 109: 2993–2999
Sandstede JJ et al. (2004) Time course of 23Na signal intensity after myocardial infarction in humans. Magn Reson Med 52: 545–551
Selvanayagam JB et al. (2004) Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation 110: 1535–1541
Elliott MD and Kim RJ (2005) Late gadolinium cardiovascular magnetic resonance in the assessment of myocardial viability. Coron Artery Dis 16: 365–372
Conway MA et al. (1991) Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 338: 973–976
Conway MA et al. (1998) Mitral regurgitation: impaired systolic function, eccentric hypertrophy, and increased severity are linked to lower phosphocreatine/ATP ratios in humans. Circulation 97: 1716–1723
Beyerbacht HP et al. (2001) Aortic valve replacement in patients with aortic valve stenosis improves myocardial metabolism and diastolic function. Radiology 219: 637–643
Scheuermann-Freestone M et al. (2003) Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107: 3040–3046
Diamant M et al. (2003) Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol 42: 328–335
Metzler B et al. (2002) Decreased high-energy phosphate ratios in the myocardium of men with diabetes mellitus type I. J Cardiovasc Magn Reson 4: 493–502
Robinson M et al. (2006) Abnormal cardiac energetics are associated with diastolic dysfunction in uncomplicated obesity. Circulation 18: 905
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hudsmith, L., Neubauer, S. Detection of myocardial disorders by magnetic resonance spectroscopy. Nat Rev Cardiol 5 (Suppl 2), S49–S56 (2008). https://doi.org/10.1038/ncpcardio1158
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio1158
This article is cited by
-
Cardiovascular magnetic resonance imaging and spectroscopy in clinical long-COVID-19 syndrome: a prospective case–control study
Journal of Cardiovascular Magnetic Resonance (2022)
-
Delayed Myocardial Enhancement in Pediatric Hypertrophic Cardiomyopathy: Correlation with LV Function, Echocardiography, and Demographic Parameters
Pediatric Cardiology (2017)
-
Cardiac PET/MRI
Current Cardiovascular Imaging Reports (2013)
-
Imaging and Modeling of Myocardial Metabolism
Journal of Cardiovascular Translational Research (2010)