Abstract
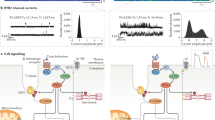
Abnormal regulation of intracellular Ca2+ by sarcoplasmic reticulum plays a part in the mechanism underlying contractile and relaxation dysfunction in heart failure (HF). The protein-kinase-A-mediated hyperphosphorylation of ryanodine receptors in the sarcoplasmic reticulum has been shown to cause the dissociation of FKBP12.6 (also known as calstabin-2) from ryanodine receptors in HF. In addition, several disease-linked mutations in the ryanodine receptors have been reported in patients with catecholaminergic polymorphic ventricular tachycardia or arrhythmogenic right ventricular cardiomyopathy type 2. The unique distribution of these mutation sites has led to the concept that the interaction among the putative regulatory domains within the ryanodine receptors has a key role in regulating channel opening. The knowledge gained from various studies of ryanodine receptors under pathologic conditions might lead to the development of new pharmacological or genetic strategies for the treatment of HF or cardiac arrhythmia. In this review, we focus on the role of the Ca2+-release channel, the ryanodine receptor, in the pathogenesis of HF and fatal arrhythmia, and the possibility of developing new therapeutic strategies for targeting this receptor.
Key Points
-
Alterations in calcium ion (Ca2+) cycling can cause depression of mechanical performance of the heart
-
The calcium-release channel, the ryanodine receptor (RyR)2, seems to have a notable role in heart failure and fatal arrhythmia
-
Hyperphosphorylation of RyRs in the sarcoplasmic reticulum leads to abnormal intracellular Ca2+ concentrations, which contribute to contractile and relaxation dysfunction in heart failure
-
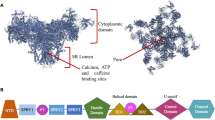
Several disease-linked mutations in the RyRs linked to regulatory domains have been implicated in the altered regulation of channel opening
-
RyR2 might prove to be a useful therapeutic target
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Braunwald E and Bristow MR (2000) Congestive heart failure: fifty years of progress. Circulation 102: IV14–IV23
Hasenfuss G and Pieske B (2002) Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34: 951–969
Yano M et al. (2005) Altered intracellular Ca2+ handling in heart failure. J Clin Invest 115: 556–564
Takeshima H et al. (1989) Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339: 439–445
Giannini G et al. (1995) The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol 128: 893–904
Nakai J et al. (1990) Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett 271: 1–2
Otsu K et al. (1990) Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem 265: 13472–13483
Marks AR et al. (2002) Regulation of ryanodine receptors via macromolecular complexes: a novel role for leucine/isoleucine zippers. Trends Cardiovasc Med 12: 166–170
Franzini-Armstrong C and Jorgensen AO (1994) Structure and development of E-C coupling units in skeletal muscle. Annu Rev Physiol 56: 509–534
Zorzato F et al. (1990) Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem 265: 2244–2256
Du GG et al. (2002) Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1). Proc Natl Acad Sci USA 99: 16725–16730
Sorrentino V and Volpe P (1993) Ryanodine receptors: how many, where and why? Trends Pharmacol Sci 14: 98–103
Bers DM (2004) Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol 37: 417–429
Marx SO et al. (2001) Phosphorylation-dependent regulation of ryanodine receptor: a novel role for leucine/isoleucine zippers. J Cell Biol 153: 699–708
Zhang L et al. (1997) Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 272: 23389–23397
Wagenknecht T et al. (1989) Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature 338: 167–170
Marks AR et al. (2002) Progression of heart failure: is protein kinase a hyperphosphorylation of the ryanodine receptor a contributing factor? Circulation 105: 272–275
Marx SO et al. (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376
Yano M et al. (2000) Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circulation 102: 2131–2136
Marks AR (2002) Ryanodine receptors, FKBP12, and heart failure. Front Biosci 7: d970–d977
Wehrens XH et al. (2003) FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113: 829–840
Marx SO et al. (2001) Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ Res 88: 1151–1158
Hu X-F et al. (2005) Depletion of FKBP does not affect the interaction between isolated ryanodine receptors. Biochem Biophys Res Commun 336: 128–133
Vest JA et al. (2005) Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 111: 2025–2032
Stange M et al. (2003) Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem 278: 51693–51702
Xiao B et al. (2004) Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6). Circ Res 94: 487–495
Wehrens XH et al. (2004) Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304: 292–296
Oda T et al. (2005) Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation 111: 3400–3410
George CH et al. (2003) Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res 93: 531–540
Valdivia HH et al. (1995) Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science 267: 1997–2000
Li Y et al. (2002) Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res 90: 309–316
Ginsburg KS and Bers DM (2004) Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol 556: 463–480
Xiao B et al. (2005) Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res 96: 847–855
Reiken S et al. (2003) β-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation 107: 2459–2466
Reiken S et al. (2001) β-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 104: 2843–2848
Doi M et al. (2002) Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation 105: 1374–1379
Pathak A et al. (2005) Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res 96: 756–766
Reiken S et al. (2003) Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J Biol Chem 278: 444–453
Obayashi M et al. (2005) Spontaneous diastolic contractions and phosphorylation of the cardiac ryanodine receptor at serine-2808 in congestive heart failure in rats. Cardiovasc Res [10.1016/j.cardiores.2005.07.010]
Jiang MT et al. (2002) Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res 91: 1015–1022
Vatner DE et al. (1994) Decrease in myocardial ryanodine receptors and altered excitation-contraction coupling early in the development of heart failure. Circulation 90: 1423–1430
Masumiya H et al. (2003) Localization of the 12.6-kDa FK506-binding protein (FKBP12.6) binding site to the NH2-terminal domain of the cardiac Ca2+ release channel (ryanodine receptor). J Biol Chem 278: 3786–3792
Zissimopoulos S and Lai FA (2005) Interaction of FKBP12.6 with the cardiac ryanodine receptor C-terminal domain. J Biol Chem 280: 5475–5485
Yano M et al. (2003) FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 107: 477–484
Brini M (2004) Ryanodine receptor defects in muscle genetic diseases. Biochem Biophys Res Commun 322: 1245–1255
McCarthy TV et al. (2000) Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum Mutat 15: 410–417
Swan H et al. (1999) Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol 34: 2035–2042
Priori SG (2001) Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103: 196–200
Laitinen PJ et al. (2001) Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 103: 485–490
Lehnart SE et al. (2004) Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation 109: 3208–3214
Jiang D et al. (2004) RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc Natl Acad Sci USA 101: 13062–13067
Cerrone M et al. (2005) Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res 96: e77–e82
Ikemoto N and Yamamoto T (2002) Regulation of calcium release by interdomain interaction within ryanodine receptors. Front Biosci 7: d671–d683
Yamamoto T et al. (2000) Postulated role of interdomain interaction within the ryanodine receptor in Ca2+ channel regulation. J Biol Chem 275: 11618–11625
Lamb GD et al. (2001) Effects of a domain peptide of the ryanodine receptor on Ca2+ release in skinned skeletal muscle fibers. Am J Physiol Cell Physiol 281: C207–C214
Shtifman A et al. (2002) Interdomain interactions within ryanodine receptors regulate Ca2+ spark frequency in skeletal muscle. J Gen Physiol 119: 15–32
Yamamoto T and Ikemoto N (2002) Peptide probe study of the critical regulatory domain of the cardiac ryanodine receptor. Biochem Biophys Res Commun 291: 1102–1108
Zhang J et al. (2003) Three-dimensional localization of divergent region 3 of the ryanodine receptor to the clamp-shaped structures adjacent to the FKBP binding sites. J Biol Chem 278: 14211–14218
Serysheva II et al. (2005) Structure of Ca2+ release channel at 14 A resolution. J Mol Biol 345: 427–431
Lehnart SE et al. (2005) Lehnart SE defective ryanodine receptor interdomain interactions may contribute to intracellular Ca2+ leak: a novel therapeutic target in heart failure. Circulation 111: 3342–3346
Thomas NL et al. (2004) Functional heterogeneity of ryanodine receptor mutations associated with sudden cardiac death. Cardiovasc Res 64: 52–60
George CH et al. (2004) Ryanodine receptor regulation by intramolecular interaction between cytoplasmic and transmembrane domains. Mol Biol Cell 15: 2627–2638
Bristow MR (2000) β-adrenergic receptor blockade in chronic heart failure. Circulation 101: 558–569
Okuda S et al. (2004) Valsartan restores sarcoplasmic reticulum function with no appreciable effect on resting cardiac function in pacing-induced heart failure. Circulation 109: 911–919
Kaneko N (1994) New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac death and intracellular calcium blocking action. Drug Dev Res 33: 429–438
Xin HB et al. (2002) Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 416: 334–338
Boyden PA et al. (2004) 2APB- and JTV519(K201)-sensitive micro Ca2+ waves in arrhythmogenic Purkinje cells that survive in infarcted canine heart. Heart Rhythm 1: 218–226
Kimura J et al. (1999) Effects of a novel cardioprotective drug, JTV-519 on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol 79: 275–281
Wehrens XH et al. (2005) Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci USA 102: 9607–9612
Acknowledgements
This work was supported by grants-in-aid for scientific research from The Ministry of Education in Japan to M Yano and M Matsuzaki, by grants from the Takeda Science Foundation to T Yamamoto and Y Ikeda, and by the Japan Heart Foundation/Pfizer Japan Inc. Grant for Research on Cardiovascular Disease to T Yamamoto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yano, M., Yamamoto, T., Ikeda, Y. et al. Mechanisms of Disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Rev Cardiol 3, 43–52 (2006). https://doi.org/10.1038/ncpcardio0419
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0419
This article is cited by
-
Enhanced Ca2+-induced Ca2+ release from intracellular stores contributes to catecholamine hypersecretion in adrenal chromaffin cells from spontaneously hypertensive rats
Pflügers Archiv - European Journal of Physiology (2015)
-
RYR3 gene polymorphisms and cardiovascular disease outcomes in the context of antihypertensive treatment
The Pharmacogenomics Journal (2013)
-
Postmortem genetic testing of the ryanodine receptor 2 (RYR2) gene in a cohort of sudden unexplained death cases
International Journal of Legal Medicine (2013)
-
Ryanodine receptors
Skeletal Muscle (2011)
-
Isoproterenol-induced FKBP12.6/12 downregulation is modulated by ETA and ETB receptors and reversed by argirhein, a derivative of rhein
Acta Pharmacologica Sinica (2011)