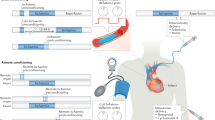
Abstract
After an acute myocardial infarction (AMI), early reperfusion by thrombolysis or primary percutaneous coronary intervention remains the most-effective strategy for limiting the size of an evolving infarct. The mortality from AMI, however, remains significant, due partly to the lethal reperfusion injury that occurs on reperfusing the ischemic myocardium. Novel cardioprotective strategies are required to target this form of injury. In ischemic preconditioning transient, nonlethal episodes of myocardial ischemia and reperfusion before the index ischemic episode reduce infarct size. The cardioprotective potential of ischemic preconditioning has not been realized in clinical practice because it necessitates an intervention applied before the onset of AMI, which is difficult to predict. A more-amenable approach to cardioprotection is to intervene at the onset of reperfusion, the timing of which is under the control of the operator. In this regard, ischemic postconditioning, in which transient episodes of myocardial ischemia and reperfusion administered at the onset of reperfusion reduce infarct size, constitutes one such intervention. Interestingly, studies suggest that ischemic preconditioning and postconditioning activate the same signaling pathway at the time of reperfusion, thereby offering a common target for cardioprotection. Therefore, the pharmacologic recruitment of this signaling pathway at the time of myocardial reperfusion might allow one to harness the cardioprotective potential of ischemic preconditioning and postconditioning. In this review, we discuss the potential application of ischemic preconditioning and postconditioning in the clinical arena of myocardial ischemia and reperfusion, and examine the common signaling pathways by which this might be achieved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Fuster V (1999) Epidemic of cardiovascular disease and stroke: the three main challenges. Presented at the 71st scientific sessions of the American Heart Association. Dallas, Texas. Circulation 99: 1132–1137
Braunwald E and Kloner RA (1985) Myocardial reperfusion: a double-edged sword? J Clin Invest 76: 1713–1719
Bolli R et al. (2004) Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134
Kloner RA and Rezkalla SH (2004) Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol 44: 276–286
Kloner RA et al. (1998) Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation 97: 1848–1867
Murry CE (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136
Zhao ZQ et al. (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H579–H588
Yellon DM and Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83: 1113–1151
Marber MS et al. (1993) Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 88: 1264–1272
Garlid KD et al. (1997) Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res 81: 1072–1082
Vanden Hoek TL et al. (1998) Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273: 18092–18098
Hausenloy DJ et al. (2002) Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res 55: 534–543
Ikonomidis JS et al. (1994) Preconditioning human ventricular cardiomyocytes with brief periods of simulated ischaemia. Cardiovasc Res 28: 1285–1291
Walker DM et al. (1995) Preconditioning in isolated superfused human muscle. J Mol Cell Cardiol 27: 1349–1357
Williams DO et al. (1985) Adaptation to the stress of tachycardia in patients with coronary artery disease: insight into the mechanism of the warm-up phenomenon. Circulation 71: 687–692
Rezkalla SH and Kloner RA (2004) Ischemic preconditioning and preinfarction angina in the clinical arena. Nat Clin Pract Cardiovasc Med 1: 96–102
Deutsch E et al. (1990) Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation 82: 2044–2051
Jenkins DP et al. (1995) Preconditioning the human myocardium: recent advances and aspirations for the development of a new means of cardioprotection in clinical practice. Cardiovasc Drugs Ther 9: 739–747
Moolman JA et al. (1997) Ischaemic preconditioning does not protect hypertrophied myocardium against ischaemia. S Afr Med J 87 (Suppl 3): C151–C156
Schulman D et al. (2001) Effect of aging on the ability of preconditioning to protect rat hearts from ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol 281: H1630–H1636
Tsang A et al. (2005) Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes 54: 2360–2364
IONA Study group (2002) Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet 359: 1269–1275
Ono H et al. (2004) Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: role of inhibitory effect on reactive oxygen species formation. Am Heart J 148: E15
Avkiran M and Marber MS (2002) Na+/H+ exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol 39: 747–753
Bolli R (2003) The role of sodium–hydrogen ion exchange in patients undergoing coronary artery bypass grafting. J Card Surg 18 (Suppl 1): 21–26
Quintana M et al. (2004) Pharmacological prevention of reperfusion injury in acute myocardial infarction. A potential role for adenosine as a therapeutic agent. Am J Cardiovasc Drugs 4: 159–167
Dana A et al. (1998) Prolonging the delayed phase of myocardial protection: repetitive adenosine A1 receptor activation maintains rabbit myocardium in a preconditioned state. J Am Coll Cardiol 31: 1142–1149
Yellon DM and Baxter GF (1999) Reperfusion injury revisited: is there a role for growth factor signaling in limiting lethal reperfusion injury? Trends Cardiovasc Med 9: 245–249
Hausenloy DJ and Yellon DM (2004) New directions for protecting the heart against ischaemia–reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res 61: 448–460
Heusch G (2004) Postconditioning: old wine in a new bottle? J Am Coll Cardiol 44: 1111–1112
Tsang A (2004) Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res 95: 230–232
Okamoto F et al. (1986) Reperfusion conditions: importance of ensuring gentle versus sudden reperfusion during relief of coronary occlusion. J Thorac Cardiovasc Surg 92: 613–620
Sato H et al. (1997) Gradual reperfusion reduces infarct size and endothelial injury but augments neutrophil accumulation. Ann Thorac Surg 64: 1099–1107
Yang XM et al. (2004) Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol 44: 1103–1110
Argaud L et al. (2005) Postconditioning inhibits mitochondrial permeability transition. Circulation 111: 194–197
Hausenloy DJ et al. (2005) The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med 15: 69–75
Downey JM and Cohen MV (2005) We think we see a pattern emerging here. Circulation 111: 120–121
Hausenloy DJ et al. (2005) Ischemic preconditioning protects by activating pro-survival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288: H971–H976
Griffiths EJ and Halestrap AP (1995) Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J 307 (Pt 1): 93–98
Hausenloy DJ and Yellon DM (2003) The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol 35: 339–341
Jonassen AK (2001) Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res 89: 1191–1198
Bell RM and Yellon DM (2003) Atorvastatin, administered at the onset of reperfusion, and independent of lipid lowering, protects the myocardium by up-regulating a pro-survival pathway. J Am Coll Cardiol 41: 508–515
Bullard AJ et al. (2005) Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol [10.1007/s00395-005-0537-4]
Bose AK et al. (2005) Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54: 146–151
Mensah K et al. (2005) Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: a potential role for phosphatase and tensin homolog deleted on chromosome ten? J Am Coll Cardiol 45: 1287–1291
Hausenloy DJ et al. (2003) Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia–reperfusion injury. Cardiovasc Res 60: 617–625
Halkos ME et al. (2004) Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg 78: 961–969
Fath-Ordoubadi F and Beatt KJ (1997) Glucose–insulin–potassium therapy for treatment of acute myocardial infarction: an overview of randomized placebo-controlled trials. Circulation 96: 1152–1156
Mehta SR et al. (2005) Effect of glucose–insulin–potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE–ECLA randomized controlled trial. JAMA 293: 437–446
Apstein CS and Opie LH (2005) A challenge to the metabolic approach to myocardial ischaemia. Eur Heart J 26: 956–959
Ehrenreich H et al. (2002) Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med 8: 495–505
Nikolaidis LA et al. (2004) Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 109: 962–965
Kin H et al. (2004) Postconditioning attenuates myocardial ischemia–reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res 62: 74–85
Galagudza M et al. (2004) Ischemic postconditioning: brief ischemia during reperfusion converts persistent ventricular fibrillation into regular rhythm. Eur J Cardiothorac Surg 25: 1006–1010
Sun K et al. (2004) Role of mitochondria in cell apoptosis during hepatic ischemia–reperfusion injury and protective effect of ischemic postconditioning. World J Gastroenterol 10: 1934–1938
Yang XM et al. (2005) Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol 100: 57–63
Chiari PC et al. (2005) Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology 102: 102–109
Serviddio G et al. (2005) Brief hypoxia before normoxic reperfusion (postconditioning) protects the heart against ischemia–reperfusion injury by preventing mitochondria peroxyde production and glutathione depletion. FASEB J 19: 354–361
Sun HY et al. (2005) Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol 288: H1900–H1908
Darling CE et al. (2005) 'Postconditioning' via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK 1/2. Am J Physiol Heart Circ Physiol [10.1152/ajpheart.00055.2005]
Kin H et al. (2005) Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res 67: 124–133
Kerendi F et al. (2005) Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol [10.1007/s00395-005-0539-2]
Acknowledgements
We thank the British Heart Foundation for continuing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yellon, D., Hausenloy, D. Realizing the clinical potential of ischemic preconditioning and postconditioning. Nat Rev Cardiol 2, 568–575 (2005). https://doi.org/10.1038/ncpcardio0346
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0346
This article is cited by
-
Remote Ischemic Postconditioning Inhibited Mitophagy to Achieve Neuroprotective Effects in the Rat Model of Cardiac Arrest
Neurochemical Research (2021)
-
Enhanced Cardiac S100A1 Expression Improves Recovery from Global Ischemia-Reperfusion Injury
Journal of Cardiovascular Translational Research (2018)
-
Optimising cardioprotection during myocardial ischaemia: targeting potential intracellular pathways with glucagon-like peptide-1
Cardiovascular Diabetology (2014)
-
Effective Neuroprotection by Ischemic Postconditioning is Associated with a Decreased Expression of RGMa and Inflammation Mediators in Ischemic Rats
Neurochemical Research (2013)
-
The NCX3 Isoform of the Na+/Ca2+ Exchanger Contributes to Neuroprotection Elicited by Ischemic Postconditioning
Journal of Cerebral Blood Flow & Metabolism (2011)